
Research of Inducing Apoptosis and Ferroptosis in Ovarian Cancer
and Its Synergistic Anticancer Significance
Qian Yang
School of Pharmaceutical Science & Technology, Tianjin University, Tianjin 300072, China
Keywords:
Ovarian Cancer, Apoptosis, Ferroptosis, Drug Resistance.
Abstract: Ovarian cancer (OC), as a highly malignant tumor of female reproductive system, has the highest mortality.
At present, in the clinical treatment of OC, although the action mechanism of some chemical drugs is clear
and the curative effect is remarkable, they are easy to result in drug resistance, which seriously hinders the
anticancer effect of chemotherapeutic drugs. In this brief article, we summarized the two cell death modes of
apoptosis and ferroptosis, discussed the correlation between apoptosis, ferroptosis and OC, and emphasized
the synergistic effect of combined application of drugs that can induce cell apoptosis and ferroptosis to deal
with the drug resistance of OC. The latest progress of this subject will help to deal with the drug resistance of
OC and expand more ideas for clinical treatment.
1 INTRODUCTION
Ovarian cancer (OC) is a malignant tumor of the
reproductive system, its mortality rate ranked the top
among North American women in cancer death.
(
Henley, 2020)
In the clinical treatment, surgery,
radiotherapy and adjuvant chemotherapy are
commonly used, and chemotherapy is widely
appeared in tumor treatment. However, drug
resistance is one of the severe problems face by
cancer patients in the process of chemotherapy
treatment. (
Emmings, 2019)
Apoptosis escape is considered one of the result
of cancer development, which promote the resistance
of tumor cells to radiotherapy and chemotherapy.
(
Skarkova, 2019)
One mechanism for drug
resistance is alterations in apoptotic molecules that
ultimately help the cell's ability to evade death.
Therefore, it is essential to restore apoptosis of OC
cells. Besides, ferroptosis, as a new form of regulated
cell death (RCD), can enhance the therapeutic effect
of chemotherapeutic drugs on tumors. (
Scott, 2012)
Therefore, ferroptosis associated with lipid reactive
oxygen species has received clinical attention and
considered as a underlying therapeutic strategy for
OC. (
Bebber, 2020)
This article focuses on two types of cell death
process known as apoptosis and ferroptosis. We
discuss recent cases of OC treatment by inducing
apoptosis and ferroptosis of OC cells and their
synergistic antitumor significance. Therefore, it is a
new strategy to cause tumor cells to cooperate with
ferroptosis and apoptosis to conquer OC. The main
pathways of apoptosis and ferroptosis together with
the relationship between ferroptosis, apoptosis and
OC are discussed to find a breakthrough for OC
therapy.
2 APOPTOSIS
Apoptosis is a biochemical process of cell
decomposition through the interaction of specific
proteins and the programmed transmission of death-
inducing signals. (
Simon, 2019)
It was reported that
some molecules contained similar B-cell lymphoma
2 (Bcl-2) homology domains (BH domains) in
protein structure, and these molecules are all
classified as Bcl-2 family molecules. (
Maji, 2018)
Bcl-2 family proteins as indicator of chemotherapy
response and prognosis in patients with advanced
OC, suggesting that Bcl-2 family proteins are closely
related to the mechanism of apoptosis escape in OC
cells. Therefore, Bcl-2 family proteins become
promising targets for OC because apoptosis of OC
cells could be regulated by Bcl-2 family, which
provided ideas for OC treatment. (
Ridder, 2021)
The
Bcl-2 family proteins were classified into three
Yang, Q.
Research of Inducing Apoptosis and Ferroptosis in Ovarian Cancer and Its Synergistic Anticancer Significance.
DOI: 10.5220/0012018700003633
In Proceedings of the 4th International Conference on Biotechnology and Biomedicine (ICBB 2022), pages 223-227
ISBN: 978-989-758-637-8
Copyright
c
2023 by SCITEPRESS – Science and Technology Publications, Lda. Under CC license (CC BY-NC-ND 4.0)
223
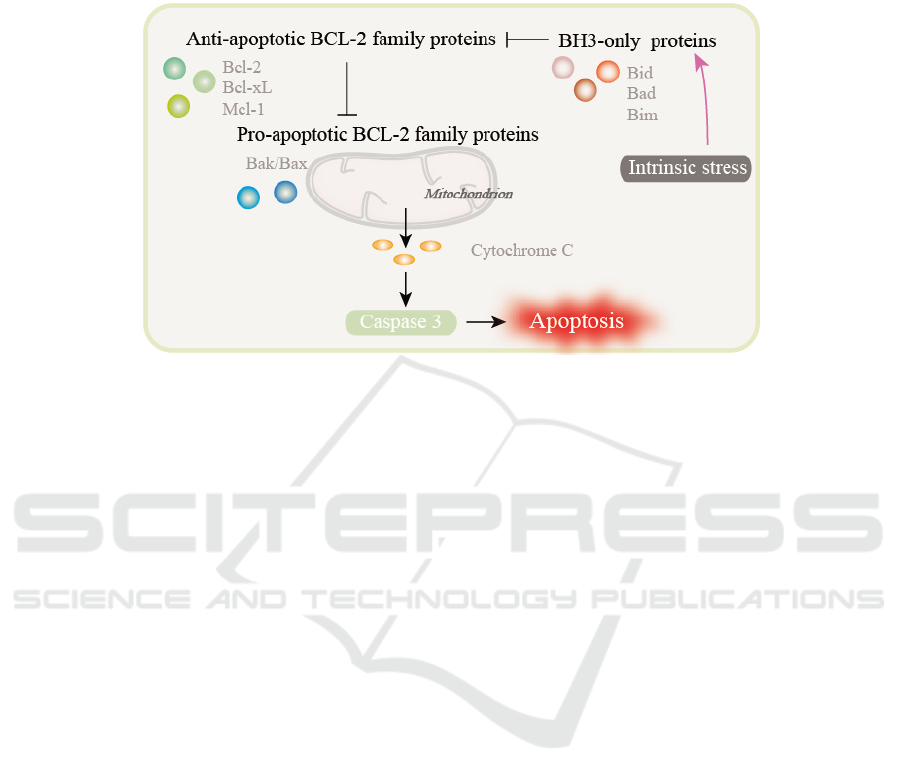
subcategories: anti-apoptotic Bcl-2 family proteins,
pro-apoptotic Bcl-2 family proteins and BH3-only
proteins. (
Basu, 2021)
(Fig.1) Many researchers
have targeted anti-apoptotic proteins to reverse the
apoptotic escape of OC cells and thus reverse drug
resistance. The anti-apoptotic family proteins include
Bcl-2, Bcl-extra long (Bcl-xL) and Myeloid leukemia
1 (Mcl-1).
Figure 1: Processes of apoptosis induced by Bcl-2 family proteins.
2.1 Regulation of BCL-2 and BCL-xL in
Ovarian Cancer
Combinations of two or more synthetic anticancer
drugs have been reported as possible alternatives for
improving efficacy and reducing the expected
toxicity and side effects of drugs. (
Xu, 2011)
Takuhei Yokoyama et al. evaluated the possible
synergistic cytotoxicity of combination therapy with
two drugs in high-grade serous OC cells. The results
showed that two drugs could induce more apoptosis
and produce synergistic cytotoxic effect on HGSOC
cells through inhibition of Bcl-2/Bcl-xL.
(
Yokoyama, 2017)
The inhibiton of Bcl-2/Bcl-xL
caused the release of Bcl-2 associated X protein
(Bax) and Bcl-2 homologous antagonist/killer
protein (Bak). Then the oligomerization of effectors
Bax and Bak caused the release of cytochrome c (cyto
c), which lead to the activation of caspases and the
induction of apoptosis. In addition, nano-targeting
systems for OC were being developed, and Farideh
Rezaie Amale et al. considered that gold
nanoparticles was a promising and appropriate
targeting system for enhancing anti-tumor activity
against OC cells. (
Amale, 2021)
2.2 Regulation of Mcl-1 in Ovarian
Cancer
At present, UMI-77 is considered to be an effective
inhibitor to reduce the activity of Mcl-1 protein, and
there are also many studies targeting Mcl-1 through
other small molecules. Qi et al. revealed the
intervention effect of apigenin on OC. (Qi, 2020)
They investigated the effect of apigenin and
determined its mechanism in the regulation of
chemotherapy resistance. The results showed that
apigenin increased the Bax/Bcl-2 ratio and by down-
regulating Mcl-1 on OC. Enhanced permeability lead
to the release of various apoptotic stimulators into the
cytoplasm, thus promoting caspases to trigger cell
apoptosis. Apigenin triggered cell death in OC cells
by enhancing mitochondria-regulated cell death and
eliminated cisplatin (DDP)-induced resistance.
3 FERROPTOSIS
Ferroptosis is a novel non-apoptotic programmed cell
death process. With the development of research, the
general pathogenesis of ferroptosis has been partially
elucidated. The general process of ferroptosis is the
direct or indirect activation of different signaling
pathways through the induction of small molecules,
thereby decreasing the activity of glutathione
peroxidase 4 (GPX4) and inhibiting the intracellular
antioxidant energy. This leads to excessive
accumulation of reactive oxygen species (ROS),
which ultimately leads to cell death. (Xie, 2016) The
occurrence of ferroptosis can be regulated by a
variety of pathways. (Fig. 2) (1) System xc- is a
cystine-glutamate reverse transporter system that
ICBB 2022 - International Conference on Biotechnology and Biomedicine
224

binds to a twelve-channel transmembrane transporter
protein SLC7A11 (xCT). Cystine and glutamate enter
cells through system xc- on the cell membrane. The
following step is the synthesis of glutathione (GSH),
the substrate of GPX4. (
Imai, 2017)
(2) Iron is
closely related to ROS accumulation and ferroptosis.
Almost all lipid peroxides can be diminished by iron
chelators, which closely connects the process of iron
metabolism with the occurrence of ferroptosis.
(
Minotti, 1987)
During iron ion oxidation, it is
oxidized to lipid ROS by Fenton reaction. (3) The
survival of all cells requires the maintenance of
membrane fluidity, and polyunsaturated fatty acids
(PUFAs) on lipid membranes are one of the factors
that maintain this fluidity. Doll et al. reported that the
acyl-CoA synthase long chain family member 4
(ACSL4) that synthesized during PUFAs oxidation
drives ferroptosis through the accumulation of
membrane phospholipids. (
Doll, 2017)
(4)
Mevalonate pathway (MVA) is a metabolic pathway
that synthesizes the precursor of steroid and terpenoid
biomolecular synthesis using acetyl coenzyme A as
raw material. (
Shimada)
Coenzyme Q10 (CoQ10)
can be produced through this pathway. As an
endogenous antioxidant, it inhibits ferroptosis by
blocking lipid peroxidation process.
3.1 The Regulation of System
xc- / GPX4 / GSH
Hong Ting et al. suggested that the cell death and
tumor suppression induced by olaparib in OC is
ferroptosis, and suggested that olaparib partially
promotes ferroptosis by inhibiting SLC7A11-
mediated GSH biosynthesis. (Hong, 2021) In
addition, Cheng Qi et.al suggested that erastin in
combination with DDP synergistically inhibits OC
cell growth and maximizes the efficacy of OC
treatment. (Cheng, 2021) When erastin was used in
combination with DDP, cell survival and the activity
of GPX4 were significantly reduced, while ROS
levels were elevated and ferroptosis occurred. Direct
targeting of GPX4 may be more effective, and Li et
al. confirmed that inhibition of GPX4 inhibited the
growth of OC cells, induced ferroptosis, reduced
Fe3+ accumulation, and inhibited lipid peroxidation
reduction ability. (Li, 2021)
3.2 The Regulation of ACSL4
ACSL4 is upregulated in a variety of cancers,
including OC, which promotes resistance to
conventional therapy through lipid metabolism
disorder. (Cui, 2018) Targeting ACSL4 could be a
key therapeutic approach for OC treatment. Ma et al.
found that ACSL4 was over-expressed in OC and
ferroptosis could be regulated in OC through
targeting ACSL4. (Ma, 2020) They demonstrated
that knockdown or over-expression of Tumor
suppressor sensitized and inhibited erastin and RSL3
induced ferroptosis in OC cells, respectively.
Figure 2: Processes of ferroptosis.
Research of Inducing Apoptosis and Ferroptosis in Ovarian Cancer and Its Synergistic Anticancer Significance
225

4 CONCLUSIONS
This article focuses on the treatment of OC by
inducing apoptosis and ferroptosis in recent years.
(Table 1) Unfortunately, there are few studies on the
synergistic treatment of OC by apoptosis and
ferroptosis. The significance of this article lies in that
it is of great significance to select the lowest effective
dose of multiple drugs for OC treatment, not only to
reduce the toxic and side effects of drugs, but also to
achieve the synergistic effect of ferroptosis and
apoptosis against cancer. Such treatment
combinations may lead to novel treatment strategies
that reduce the recurrence or drug resistance in order
to increase the sensitivity of OC to drugs.
Table 1: Summary of targets that promote cell death in ovarian cancer.
Mode of cell
death
Targets Cell lines Rf.
Apoptosis Bcl-2/Bcl-xL OVCAR3; OVCAR8; OV90 11
Apoptosis Bcl-2 A2780; HEK-293 12
Apoptosis Mcl-1 SKOV3; SKOV3/DPP 13
Ferroptosis SLC7A11 HEY; A2780 19
Ferroptosis GPX4
A2780; SKOV3; OVCA433;
OVCAR5; OVCAR8; HEY;
HOSE
p
iC
21
Ferroptosis ACSL4 HO8910; SKOV3 23
REFERENCES
A. Basu, The interplay between apoptosis and cellular
senescence: Bcl-2 family proteins as targets for
cancer therapy, Pharmacol Ther, (2021) 107943.
C.M. Bebber, F. Muller, L. Prieto Clemente, J. Weber,
S. von Karstedt, Ferroptosis in Cancer Cell Biology,
Cancers, 12 (2020).
D. Li, M. Zhang, H. Chao, Significance of glutathione
peroxidase 4 and intracellular iron level in ovarian
cancer cells— “utilization” of ferroptosis
mechanism, Inflammation Research, 70 (2021)
1177-1189.
E. Emmings, S. Mullany, Z. Chang, C.N. Landen, S.
Linder, M. Bazzaro, Targeting Mitochondria for
Treatment of Chemoresistant Ovarian Cancer,
International Journal of Molecular Sciences, 20
(2019).
F. Rezaie Amale, S. Ferdowsian, S. Hajrasouliha, R.
Kazempoor, A. Mirzaie, M. Sedigh Dakkali, I.
Akbarzadeh, S. Mohammadmahdi Meybodi, M.
Mirghafouri, Gold nanoparticles loaded into
niosomes: A novel approach for enhanced antitumor
activity against human ovarian cancer, Advanced
Powder Technology, 32 (2021) 4711-4722.
G. Minotti, S.D. Aust, The role of iron in the initiation
of lipid peroxidation, Chemistry and Physics of
Lipids, 44 (1987) 191-208.
H. Imai, M. Matsuoka, T. Kumagai, T. Sakamoto, T.
Koumura, Lipid Peroxidation-Dependent Cell Death
Regulated by GPx4 and Ferroptosis, in: S. Nagata,
H. Nakano (Eds.) Apoptotic and Non-apoptotic Cell
Death, Springer International Publishing, Cham,
2017, pp. 143-170.
I. de Ridder, M. Kerkhofs, S.P. Veettil, W. Dehaen, G.
Bultynck, Cancer cell death strategies by targeting
Bcl-2's BH4 domain, Biochimica et Biophysica Acta
(BBA) - Molecular Cell Research, 1868 (2021)
118983.
L.L. Ma, L. Liang, D. Zhou, S.W.J.N. Wang, Tumor
suppressor miR-424-5p abrogates ferroptosis in
ovarian cancer through targeting ACSL4, 68 (2020).
Q. Cheng, L. Bao, M. Li, K. Chang, X. Yi, Erastin
synergizes with cisplatin via ferroptosis to inhibit
ovarian cancer growth in vitro and in vivo, Journal
of Obstetrics and Gynaecology Research, 47 (2021)
2481-2491.
Scott J. Dixon, Kathryn M. Lemberg, Michael R.
Lamprecht, R. Skouta, Eleina M. Zaitsev, Caroline
E. Gleason, Darpan N. Patel, Andras J. Bauer,
Alexandra M. Cantley, Wan S. Yang, B. Morrison,
Brent R. Stockwell, Ferroptosis: An Iron-Dependent
Form of Nonapoptotic Cell Death, Cell, 149 (2012)
1060-1072.
Simon, Mathis, Knig, Vendela, Rissler, Thilde,
Terkelsen, Matteo, Lambrughi, E.J.P.c. biology,
Alterations of the interactome of Bcl-2 proteins in
breast cancer at the transcriptional, mutational and
structural level, 15 (2019) e1007485.
S.J. Henley, E.M. Ward, S. Scott, J. Ma, R.N. Anderson,
A.U. Firth, C.C. Thomas, F. Islami, H.K. Weir, D.R.
Lewis, R.L. Sherman, M. Wu, V.B. Benard, L.C.
Richardson, A. Jemal, K. Cronin, B.A. Kohler,
Annual report to the nation on the status of cancer,
part I: National cancer statistics, 126 (2020) 2225-
2249.
S. Doll, B. Proneth, Y.Y. Tyurina, E. Panzilius, S.
Kobayashi, I. Ingold, M. Irmler, J. Beckers, M.
Aichler, A. Walch, H. Prokisch, D. Trümbach, G.
ICBB 2022 - International Conference on Biotechnology and Biomedicine
226

Mao, F. Qu, H. Bayir, J. Füllekrug, C.H. Scheel, W.
Wurst, J.A. Schick, V.E. Kagan, J.P.F. Angeli, M.
Conrad, ACSL4 dictates ferroptosis sensitivity by
shaping cellular lipid composition, Nature Chemical
Biology, 13 (2017) 91-98.
Shimada, Kenichi, Skouta, Rachid, Kaplan, Anna, Yang,
S. Wan, Hayano, Miki, Global survey of cell death
mechanisms reveals metabolic regulation of
ferroptosis.
S. Maji, S. Panda, S.K. Samal, O. Shriwas, R. Rath, M.
Pellecchia, L. Emdad, S.K. Das, P.B. Fisher, R.
Dash, Chapter Three - Bcl-2 Antiapoptotic Family
Proteins and Chemoresistance in Cancer, in: K.D.
Tew, P.B. Fisher (Eds.) Advances in Cancer
Research, Academic Press2018, pp. 37-75.
T. Yokoyama, E.C. Kohn, E. Brill, J.-M. Lee, Apoptosis
is augmented in high-grade serous ovarian cancer by
the combined inhibition of Bcl-2/Bcl-xL and PARP,
Int J Oncol, 50 (2017) 1064-1074.
T. Hong, G. Lei, X. Chen, H. Li, X. Zhang, N. Wu, Y.
Zhao, Y. Zhang, J. Wang, PARP inhibition promotes
ferroptosis via repressing SLC7A11 and synergizes
with ferroptosis inducers in BRCA-proficient
ovarian cancer, Redox Biology, 42 (2021) 101928.
X. Cui, J. Xuan, Y. Qin, C. Long, Z.J.O. Jing, Systematic
analysis of gene expression alterations and clinical
outcomes of STAT3 in cancer, 9 (2018) 3198-3213.
V. Skarkova, V. Kralova, B. Vitovcova, E. Rudolf,
Selected Aspects of Chemoresistance Mechanisms
in Colorectal Carcinoma—A Focus on Epithelial-to-
Mesenchymal Transition, Autophagy, and
Apoptosis, Cells, 8 (2019).
Y. Xu, Y. Xin, Y. Diao, C. Lu, Z.J.P.O. Yin, Synergistic
Effects of Apigenin and Paclitaxel on Apoptosis of
Cancer Cells, 6 (2011) e29169.
Y. Qi, Z. Ding, Y. Yao, F. Ren, M. Yin, S. Yang, A.
Chen, Apigenin induces apoptosis and counteracts
cisplatin-induced chemoresistance via Mcl-1 in
ovarian cancer cells, Exp Ther Med, 20 (2020) 1329-
1336.
Y. Xie, W. Hou, X. Song, Y. Yu, J. Huang, X. Sun, R.
Kang, D. Tang, Ferroptosis: process and function,
Cell Death & Differentiation, 23 (2016) 369-379.
Research of Inducing Apoptosis and Ferroptosis in Ovarian Cancer and Its Synergistic Anticancer Significance
227
