
Therapeutic Monoclonal Antibodies: Clinical Applications
Yanjie Sun
1,*,†
, Zhuoyun Wan
2,†
and Zixiao Zhang
3,†
1
Department of Medicine, HenanVocational College of Nursing, Anyang, 455000, China
2
Clark University, Worcester, MA01610, U.S.A.
3
School of Management,
Tianjin University of Technology, Tianjin, 300382, China
Keywords:
Monoclonal Antibodies, Clinical Applications, Cancer Treatment, SARS-Cov-2.
Abstract:
Diagnostic and therapeutic monoclonal antibodies (mAbs) have a broad application prospect, conducive to
mass production by optimizing the quality of monoclonal antibodies and improving productivity. The number
of monoclonal antibodies approved for the treatment and use has increased significantly over the years. Some
improvements and modifications have been made given monoclonal antibody’s side effects and limitations.
These improvements facilitate monoclonal antibodies in various therapeutic applications, from treating
noncontagious diseases such as cancer, like breast cancer, to using monoclonal antibodies to cure infectious
diseases caused by viruses, such as viruses Covid-19 and its causing agent SARS-CoV-2. This paper reviews
monoclonal antibody’s clinical application, focusing on these two main categories and discussing its potential
future development trend.
1 INTRODUCTION
Monoclonal antibodies are homologous antibodies
produced by single clone hybridoma cells that only
recognize a specific epitope. Monoclonal antibodies
have the properties of general antibodies; monoclonal
antibodies are a type of globulin produced by the
proliferation and differentiation of B cells into plasma
cells. Such as pathogens) specifically bind, and exert
an immune effect under the participation of other
immune molecules and cells. In addition to the
general properties of antibodies, monoclonal
antibodies also have their particularities. Monoclonal
antibodies have mouse immune spleen cells (B cells)
and immortal myeloma cells fused together to form a
hybridoma cell. This hybridoma cell not only has the
ability to reproduce indefinitely but also has the
ability to secrete antibodies. Therefore, after in vitro
culture, antibodies can be secreted indefinitely.
Clinically, such as the application of diphtheria
exotoxin monoclonal antibody to treat
Corynebacterium diphtheriae; the application of anti-
endotoxin lipid A monoclonal antibody to treat
G~bacterial sepsis, etc., using a monoclonal antibody
as an affinity column can separate and purify the
†
These authors contributed equally
content of extremely low solubility Antigens such as
hormones, cytokines, and tumor antigens that are
difficult to purify; open up a new way for substance
purification; prepared monoclonal antibodies to
recognize specific receptors on the cell surface, and
couple anti-tumor drugs (such as toxins or radioactive
substances). Connect to it to build biological missiles
to overcome human diseases-tumors.
Monoclonal antibodies are vital in tumor
diagnosis and treatment. It is of great significance to
use new secretory antigens that can promote tumor
growth or metastasis as antibody blocking targets
(Scott, 2012). Monoclonal antibody-targeted therapy
for tumors is: monoclonal antibodies against tumor
antigens are attached to chemotherapy or
radiotherapy agents, and use the targeting effect of the
monoclonal antibody to carry the drug or
radiotherapy substance to the target organ and
directly kill the target cell. In addition, radio
immunoimaging can be realized to aid tumor
diagnosis by linking the radio markers with
monoclonal antibodies and applying them to patients.
Although monoclonal antibodies against tumor-
specific antigens remain to be studied, monoclonal
antibodies against tumor-associated antigens such as
alpha-fetoprotein, tumor basic protein and
Sun, Y., Wan, Z. and Zhang, Z.
Therapeutic Monoclonal Antibodies: Clinical Applications.
DOI: 10.5220/0012020800003633
In Proceedings of the 4th International Conference on Biotechnology and Biomedicine (ICBB 2022), pages 335-342
ISBN: 978-989-758-637-8
Copyright
c
2023 by SCITEPRESS – Science and Technology Publications, Lda. Under CC license (CC BY-NC-ND 4.0)
335

carcinoembryonic antigen have been used in clinical
trials for a long time. With the application of
lymphocytic hybridoma technology, many
hybridoma cell lines resistant to human tumor
markers have been established. Radionuclide-labeled
monoclonal antibodies can be used for in vivo
diagnosis, and combined with X-ray tomography
technology, it can make a quantitative diagnosis of
the size of the tumor and its metastasis. At present,
monoclonal antibodies have their limitations in the
diagnosis and treatment of tumors. Monoclonal
antibodies are mainly murine-derived antibodies,
which are satisfactory as in vitro diagnostic reagents
(Abès, 2011). If murine-derived monoclonal
antibodies are used in humans as biological
preparations, the heterogeneous proteins in the serum
of heterogeneous animals can cause allergic reactions
and even life-threatening (Marabelle, 2015). Human
monoclonal antibodies or humanized antibodies are
important for disease treatment. Although there are
reports that the culture of these cell lines is very
unstable, the human chromosomes in the fusion cells
are often selectively lost, making cell lines difficult to
cultivate. Maintain cultivation. Therefore, the
preparation of human monoclonal antibodies is a
problem that needs to be solved urgently, but no
significant progress has been made in this regard.
Prospects Various preparation technologies of
monoclonal antibodies are changing with each
passing day, but they still have their own advantages
and disadvantages and require resource integration
(Jahanshahlu, 2020). The library of antibody library
technology can be derived from immunized wild-type
or transgenic animals, or it can be derived from a
single B cell sorted out from a patient who has
recovered from a specific disease. In short, several
preparation techniques can be used interchangeably
(Cruz-Teran, 2021).
This article mainly describes the progress and
problems of monoclonal antibodies in tumor
diagnosis and treatment and new coronavirus vaccine
research. With the continuous progress and
development of research technology, monoclonal
antibody is expected to achieve a breakthrough in
tumor diagnosis and treatment, and will also be better
applied to the development of vaccines against new
coronaviruses (Anti-SARS-CoV-2 neutralizing
monoclonal antibodies: clinical pipeline – PubMed,
2021).
2 CLINICAL APPLICATIONS: IN
CANCER TREATMENT
Antibody-based cancer treatment has grown well-
established over the last 20 years, and it is currently
one of the most effective and critical techniques for
treating people with solid tumors and blood cancers
(Bayer, 2019).
2.1 For Oncology Indications, Around
30 mAbs Have Been Received
A wide variety of new therapeutic antibodies are
being evaluated in early and late-stage clinical
studies. When compared to traditional
chemotherapeutic drugs, most antibodies that have
been authorized have different, and frequently lesser
side effects.
Chemo therapies based on monoclonal antibodies
(mAbs) began to appear.
By selectively binding an antigen on a malignant
cell, mAbs might decrease non-specific toxic effects,
identify malignant cells, and either change cellular
signaling pathways toward a therapeutic outcome or
activate an immune system response against with the
cancer cell.
Antibody-drug conjugates (ADCs) are an immune
conjugation made up of a cytotoxic medication (the
payload) linked to a monoclonal antibody via a
biochemical linker. The ADC is intended to
specifically convey the ultra-toxic load direct to the
tumor cells or tissues (Chau, 2019).
Five ADCs have been approved for use in the
market, while more than 100 are being studied at
various levels of clinical testing.
The ADC is now being used in breast cancer
therapy in a variety of forward ways. This Review
will use triple-negative breast cancer (TNBC) as an
example to present the properties of mAbs therapy.
TNBC lacks the expression of progesterone receptor,
estrogen receptor (ER) and human epidermal growth
factor receptor 2 (HER2), and belongs to the invasive
breast cancer subtype. Traditional and growth factor
receptor or endocrine targeted therapies do not work
for women with TNBC. The predominant therapeutic
technique for patients is a mix of radiotherapy,
surgical intervention, and systemic chemo.
These therapeutic methods provide little clinical
benefit, and varieties of side effects such as
neutropenia and cardiotoxicity are common. Even
with therapy, initial TNBC cancers often distant
organ metastasis such as the the brain and lungs,
causing the low overall survival seen in TNBC
ICBB 2022 - International Conference on Biotechnology and Biomedicine
336

patients. Small molecule antagonists targeting DNA
double strand damage repair pathways in TNBC have
demonstrated to be a potential option for enhancing
TNBC survival rates. The FDA has authorized two
poly ADP-ribose polymerase (PARP) antagonists,
talazoparib and olaparib, for the therapy of HER2
negative later-stage cancer cases containing germ line
changes in the breast tumor vulnerability gene 1/2
(BRCA1/2).
The progression-free survival (PFS) was 7 months
for TNBC patients undergoing olaparib, compared to
4.2 months for patients who received single-agent
chemo (ClinicalTrials.gov Identifier NCT02000622).
In compared to chemotherapy- treated clients, PFS
for TNBC patients undergoing talazoparib was
prolonged by around 90 days (ClinicalTrials.gov
Identifier NCT01945775).
Though PARP inhibitors exhibited therapeutic
effectiveness as targeted therapies for TNBC, client
survival improved only marginally. Taken all
together, Additional treatments are desperately
required, which suited to the therapy of TNBC, to
stop the invasive disease's fast clinical progression.
Current cancer immunotherapy research on the
field of meeting the unmet clinical need in TNBC
presents a significant possibility. In the realm of
TNBC, programmed death-ligand 1 (PD-L1) is a
famous regulatory molecule that has recently
attracted consideration as a prospective immuno-
biological cancer therapy target.
The atezolizumab with the chemotherapeutic drug
nab-paclitaxel, an anti-PD-L1 monoclonal antibody,
the FDA authorized the conjunction for the therapy of
locally advanced, PD-L1positive unresectable, or
metastatic TNBC. The TNBC patient,whose median
overall survival for PD-L1 positive was
approximately 10 months longer than for those taking
a placebo plus nab-paclitaxel , received
atezolizumab with nab-paclitaxel.
After that, sacituzumab govitecan, the first ADC
(antibody–drug conjugate), was approved for the
therapy of metastatic TNBC. These ground-breaking
approvals have paved the way for a new era of
immunotherapy in TNBC (Dees, 2021). 86%
Bispecific antibody treatment strategies in the clinical
pipeline, which are authorized for oncology
treatment, reached. The progress of DNA
recombinant technology has led in the manufacturing
of a plethora of cancer-targeting bispecific antibodies
in a variety of configurations.
A bispecific antibody, conventional bivalent IgG-
like, produced employing knobs into holes
technology, has a fragment crystallizable region (Fc
region) which is suitable of regulating regulatory
activities, as well as fragment antigen-binding (Fab)
regions which can detect and recognize multiple
antigens. Even though some bispecific antibody
models include a muted Fc region, there are certain
bispecific antibody forms that do not have an Fc
region at all.
Diabody, dual-affinity retargeting (DART),
bispecific killer cell engager, and bispecific T cell
engager (BiTE) are examples of Fab-based bispecific
antibody constructions. The predominantly sum of
bispecific antibodies in medical studies for cancer
therapy have a process of the dual interaction of
cancer cells and immune cells, and are usually
structured as BiTEs. The blinatumomab, for
bispecific antibody development has created a
medical precedent in cancer treatment, which is a
treatment of B cell tumors situated the first-in-class
BiTE.
2.2 Therapeutic Monoclonal
Antibodies (mAbs) Targeting the
ERBB Family of Proteins Have
Shown to Be Effective in Patients
with Solid Tumors
There are many types of tumor-associated antigens
that therapeutic mAbs detect. CD30, CD20, CD52,
and CD33 are hematopoietic differentiation antigens,
which are glycoproteins that are normally linked with
a CD grouping. Both malignant cells and Normal
include cell surface differentiation antigens, which
are a broad array of carbohydrates and glycoproteins.
Growth factors receptors and growth factor are often
antigens engaged in differentiation signaling and
growth.
United therapy with immune checkpoint
suppressant, and a mass of TNBC targets such as
Ephrin receptor A10 (EphA10), Trophoblast Cell-
Surface Antigen 2 (Trop2), carcinoembryonic-
antigen-related cell-adhesion molecule 5 (CEACAM
5), Epithelial cell adhesion molecule (EpCAM), P-
cadherin, mesothelin, and EGFR have all been
integrated into immune cell-redirecting bispecific
antibody constructions. Furthermore, several
receptors on TNBC cells, such as EGFR, Notch and
HER3, can be recognized by bi-specific antibodies.
Bispecific antibodies have been developed as
potential therapies for malignant tumors by
transferring the cytotoxic effects and load of the
immune system to cancer cells, or by simultaneously
activating two functional receptors of a tumor cell. A
fab - like bispecific antibody was found to participate
in CD16 (FcRIII). A typical antibody-dependent
cytotoxicity (ADCC) mechanism is triggered when a
Therapeutic Monoclonal Antibodies: Clinical Applications
337

stimulating receptor CD16, widely observed on NK
cells, connects to the Fc region of an antibody
conjugated to a target antigen.
Human epidermal growth factor receptor 3
(HER3), extracellular signal - regulated tyrosine
kinase, and also another a bispecific diabody-Fc
integration protein marking EGFR found on TNBC
cells, was recently developed. The assay methods of
cell culture in vitro monolayer and more complicated,
physiologically associated 3D perfect sphere models
proved that the EGFR ×HER3 bispecific antibody
effectively inhibited TNBC cellular proliferation.
Furthermore, in an murine models with orthotic
MDA-MB-468 TNBC, the EGFR× HER3 bispecific
suppressed growth and the survival of TNBC cancer
stem cells (CSCs).
Positively, HER3 and Multi-specific EGFR
identification has shown clinical effectiveness and
safety in other cancers like as colorectal cancer and
head and neck cancer identifier. In some other
research, dual HER3 and EGFR blockade has been
seen to increase TNBC cell sensitivity to
phosphatidylinositol 3-kinase (PI3K) inhibitors,
revealing the need for more research into combination
treatment for TNBC.
Interestingly, EGFR Notch bispecific antibodies
augmented the response to therapy of TNBC cells to
PI3K inhibition, as demonstrated by a significant
decrease in TNBC CSC communities. The same
phenomenon was found with EGFR × Notch
bispecific antibodies.
Recently, an innovative technique for delivering
immune response therapeutic approaches to TNBC
cancers using lipid-coated phosphate and calcium
nanoparticles was established (LCP NPs). The LCP
NPs were not only designed and synthesized on the
outside with a PEG × EGFR bispecific antibody but
was also added with cell apoptosis siRNA and
indocyanine green on the inside of nanoparticle.
Consequently, LCP NPs chemically modified
with PEG × EGFR bispecific antibodies function in
TNBC cancer cells with EGFR-expressing, when
exposed to near-infrared radioactivity in vitro, and
removed TNBC tumors completely and induced cell
death in TNBC cells in vivo. One study shows that
bispecific antibodies can be used in photothermal
therapy/a gene therapy-based nanoparticle platform
to cure TNBC tumors.
Table 1: Tumor-associated antigens targeted by therapeutic monoclonal antibodies.
Antigen
cate
g
or
y
Examples of
anti
g
ens
Examples of therapeutic mAbs
raised a
g
ainst these tar
g
ets
Tumor types expressing antigen
Haematopoietic
differentiation
antigens
CD20
Rituximab Non-Hod
g
kin’s l
y
m
p
homa
Ibritumomab tiuxetan and
tositumomab
Lymphoma
CD30 Brentuximab vedotin Hod
g
kin’s l
y
m
p
homa
CD33 Gemtuzumab ozogamicin Acute myelogenous leukemia
CD52 Alemtuzumab Chronic l
y
m
p
hoc
y
tic leukemia
Glycoproteins
expressed by
solid
tumors
EpCAM IGN101 an
d
adecatumumab Epithelial tumors (breast, colon an
d
lung)
CEA Labetuzumab Breast, colon an
d
lun
g
tumors
gpA33 huA33 Colorectal carcinoma
Mucins Pemtumomab an
d
ore
g
ovomab Breast, colon, lun
g
an
d
ovarian tumors
TAG-72 CC49 (minretumomab) Breast, colon an
d
lung tumors
CAIX cG250 Renal cell carcinoma
PSMA J591 Prostate carcinoma
Folate-binding
protein
MOv18 and MORAb-003
(farletuzumab)
Ovarian tumors
Glycolipids
Gangliosides
(such as GD2,
GD3 and
GM2
)
3F8, ch14.18 and KW-2871
Neuroectodermal tumors and some
epithelial tumors
Carbohydrates Le
y
hu3S193 an
d
IgN311 Breast, colon, lung an
d
p
rostate tumors
Targets of anti-
angiogenic
mAbs
VEGF Bevacizumab Tumo
r
vasculature
VEGFR IM-2C6 an
d
CDP791 Epithelium-derive
d
soli
d
tumors
Integrin αVβ3
Etaracizumab Tumor vasculature
Integrin
αVβ31
Volociximab Tumor vasculature
Growth and
differentiation
EGFR
Cetuximab, panitumumab,
nimotuzumab an
d
806
Glioma, lung, breast, colon, and head and
nec
k
tumors
ICBB 2022 - International Conference on Biotechnology and Biomedicine
338

signaling
ERBB2 Trastuzumab and pertuzumab
Breast, colon, lung, ovarian and prostate
tumors
ERBB3 MM-121
Breast, colon, lung, ovarian and prostate,
tumors
MET
AMG 102, METMAB and SCH
900105
Breast, ovary and lung tumors
IGF1R
AVE1642, IMC-A12, MK-0646,
R1507
an
d
CP 751871
Glioma, lung, breast, head and neck, prostate
and
thyroi
d
cance
r
EPHA3
KB004 and IIIA4
Lung, kidney and colon tumors, melanoma,
glioma
an
d
haematolo
g
ical mali
g
nancies
TRAILR1 Mapatumumab (HGS-ETR1)
Colon, lung and pancreas tumors and
haematolo
g
ical mali
g
nancies
TRAILR2 HGS-ETR2 and CS-1008
RANKL Denosumab Prostate cance
r
an
d
b
one metastases
Stromal and
extracellular
matrix
anti
g
ens
FAP Sibrotuzumab and F19
Colon, breast, lung, pancreas, and head and
neck
tumours
Tenascin 81C6 Glioma,
b
reast an
d
p
rostate tumors
CAIX, carbonic anhydrase IX; CEA, carcinoembryonic antigen; EGFR, epidermal growth factor receptor; EpCAM,
epithelial cell adhesion molecule; EPHA3, ephrin Receptor A3; FAP, fibroblast activation protein; gpA33, glycoprotein
A33; IGF1R, insulin-like growth factor 1 receptor; Le
y
, Lewis Y antigen; mAbs, monoclonal antibodies; PSMA,
prostate-specific membrane antigen; RANKL, receptor activator of nuclear factor-kB ligand; TAG-72, tumor-associated
glycoprotein 72; TRAILR, tumor necrosis factor-related apoptosis-inducing ligand receptor; VEGF, vascular endothelial
g
rowth factor; VEGFR, VEGF rece
p
tor.
3 CLINICAL APPLICATIONS: IN
INFECTIOUS DISEASES
As of recent years, at least 20 neutralizing
monoclonal antibodies (mAbs) medicines are now in
late-stage clinical studies or have been permitted for
use in treating a variety of transmittable diseases,
including influenza viruses, Ebola, respiratory
syncytial viruses (RSV), and others (Wrapp, 2020).
Targeting SARS-CoV-2 as the causative agent of
COVID-19 has been a hot research topic since the
pandemic. Monoclonal antibodies against SARS-
CoV-2 have the potential to be employed for both
infection prevention and treatment. SARS-CoV and
MERS-CoV monoclonal antibodies have already
been shown to be beneficial in animal models
(Taylor, 2021).
There were no approved COVID-19 preventative
vaccinations or therapies when the outbreak began.
Blocking mAbs are among the finest candidates for
neutralizing viral infection due to their exceptional
antigen specificity. The researchers aimed to locate
and manufacture blocking monoclonal antibodies
from memory B-cell libraries of newly cured
individuals to counteract the virus from entering
healthy host cells. Similar to SARS-CoV, which
caused an outbreak of SARS in 2002-2004, SARS-
CoV-2 also uses a high-glucose homologous trimer
spike (S) protein to bind to receptors and virus entry
(Zhou, 2020). Two subunits, S1 and S2, constitute the
S protein of SARS-CoV-2 and undergo drastic
conformational changes that expose the critical
residues of the receptor-binding domain (RBD)
binding to the receptor, thereby enabling the binding
of the host cell receptor human angiotensin-
converting enzyme 2 (hACE2), which SARS-CoV
and SARS-CoV-2 share. S protein is metastable, and
RBD binding to the hACE2 receptor may promote
S2-mediated viral host membrane blending and viral
entrance, as S1 protein is detached from S2 protein.
RBD is an exposed mark for neutralizing antibodies
because it is involved in SARS-CoV-2 access into
healthy host cells. An S1-targeting monoclonal
antibody made from genetically modified mice
immunized with the expression of human Ig
differential chains has recently been shown to
counterbalance SARS-CoV-2 and SARS-CoV
contagion; it is, however, neutral of the RBD-HACE2
connection being blocked due to unknown reasons
(Cohen, 2021).
The researchers primarily looked for antibodies to
SARS-CoV-2 S1 protein IgG in blood serum from
individuals who had lately survived from COVID-19.
Using an enzyme-linked immunosorbent assay
(ELISA), they discovered that the majority of the 26
recovered COVID-19 patients were capable of
Therapeutic Monoclonal Antibodies: Clinical Applications
339
producing a high quantity of SARS-CoV-2 S1-
specific IgG antibodies. Relatively low anti-S1 IgG
antibody responses were only found in 3 patients.
They also discovered that SARS-CoV-2 IgG
antibodies explicit to RBD were detected in all of the
affected individuals. Researchers successfully
manufactured two human neutralizing monoclonal
antibodies utilizing SARS-CoV-2 RBD-specific
memory B cells obtained from patients who
recovered from the disease. These two monoclonal
antibodies attach to SARS-CoV-2 RBD and disrupt
the connection between SARS-CoV-2 RBD and the
hACE2 receptor, thereby neutralizing SARS-CoV-2
S protein pseudovirus contamination (Chen, 2020).
Passive vaccination uses antigen-specific
monoclonal or polyclonal antibodies derived from
animal or human blood sources. Two significant
uncertainties in passive immunization may also exist
in neutralizing monoclonal antibodies: first, whether
they could potentially affect long-term physical
immunity as prevention or treatment. It is unclear if
the existence of circulating neutralizing monoclonal
antibodies affects protective immunity via infection
memory or vaccination, given the high doses used and
antibody half-lives. Rodent and primate models of
RSV infection suggest that passive antibody transfer
does reduce the development of the recipient’s human
immunity. Nevertheless, long-term memory is still
adequate to defend the host from second-time
reinfection, thanks to the presence of an undamaged
T-cell memory chamber (Marovich, 2020). Second,
will the mutation of resistant virus affect the
therapeutic effectiveness? Clinical evidence suggests
that SARS-CoV-2 spikes (S) protein mutations can
evade polyclonal serum, resulting in lower
convalescent plasma neutralization activity against
particular viral variants. As a result, monoclonal
antibodies may require a mixture therapy of
monoclonal antibodies to boost clinical efficacy and
prevent treatment failure in the future, depending on
the source of infection and the targeted epitope
(Crowe, 2001).
It is essential to notice that Taylor also proposed
that “due to the vast number of persons sick and the
high intensity of virus transmission among humans,
the COVID-19 pandemic poses a bigger risk of
escape mutations than the Ebola outbreak did”, which
was later approved by the case of Delta variant and
Omicron variant.
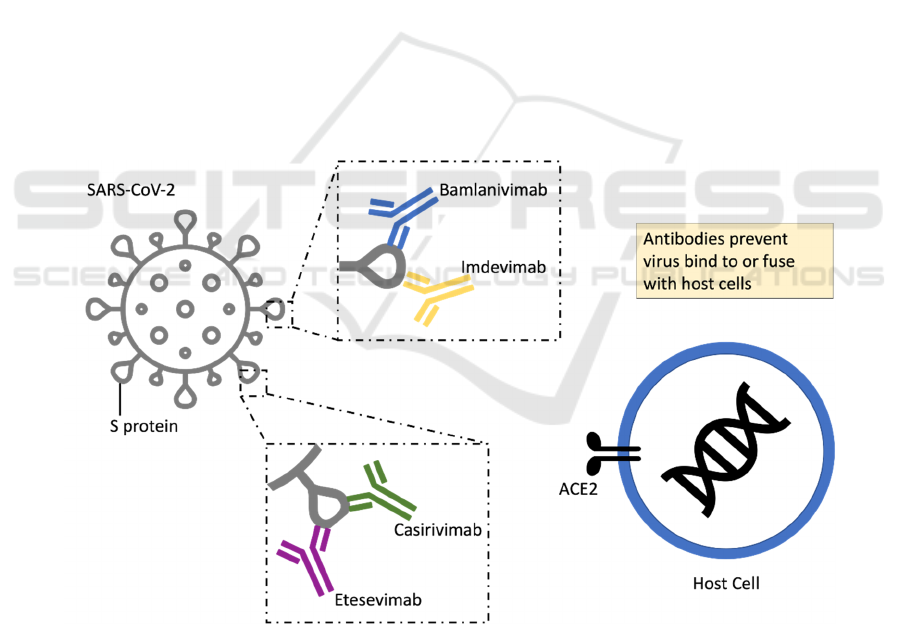
Figure 1: Neutralizing mAbs inhibit SARS-CoV-2 by binding to the spike (S) protein.
RT-PCR as a proxy for viral infection or viral
replication scale for SARS-CoV-2. According to the
findings, the monoclonal antibody serves as an
antiviral agent, reducing viral load in the
nasopharynx. The effect of monoclonal antibodies
and other medications on viral load could be a crucial
criterion for designing therapies to treat early
COVID-19 infections (Crowe, 2001).
An effective vaccine is necessary to cure the
epidemic. The functional and active evaluation of
several COVID-19 vaccine candidates is expected to
shorten the vaccine development process from years
ICBB 2022 - International Conference on Biotechnology and Biomedicine
340

or even decades to 12 to 18 months. Monoclonal
antibodies offer another way to prevent COVID-19.
Passive monoclonal antibody infusion as a
prophylactic treatment before or after exposure
provides immediate protection from an infection that
can last weeks or even months. To extend the possible
protection, the new technique that can modify the
antibody Fc region to extend the half-life of the
monoclonal antibody by up to several months,
depending on the concentration of the desired
monoclonal antibody. Even after an uninfected
person has been vaccinated, the benefits of passive
immunization can be seen during the period it will
take for the immune system to establish an immune
response using the mRNA information carried by the
vaccine. Neutralizing monoclonal antibodies is
especially helpful in health care facilities, homes, and
kindergartens where people with low immunity
gather because outbreaks are common and
devastating. During epidemics, nursing home patients
are given monoclonal antibodies. They may help slow
the disease’s course during early infections that go
untreated. Furthermore, the elderly and those with
underlying problems may not have a sturdy protective
response to vaccination, necessitating the use of
monoclonal antibodies to give protection (Taylor,
2021).
4 CONCLUSION
The attention of therapeutic monoclonal antibodies is
increasing year by year. Their high specificity for
antigen can offer various applicable medical
treatments, and the emergence of molecularly
targeted drugs has made the growth of a new
generation of therapeutic medications possible. In this
review paper, human monoclonal antibodies are
introduced as homologous antibodies produced by
single clone hybridoma cells, produced by the
proliferation and differentiation of B cells. The
clinical application of mAb therapies can be separated
into two main categories, cancer treatment, and
infectious diseases medicine. The most successful
class of antibodies targeting the ERBB family is the
usage of therapeutic mAbs on patients with solid
tumors, and one of the examples in breast cancer.
Focusing on treating infectious diseases, the efficacy
of mAb therapies targeting SARS-CoV-2 was
discussed as the causative agent of Covid-19 and its
advantage compared to CPT. In future studies of mAb
therapies targeting SARS-CoV-2, several
combination therapies are under clinical trials, like
bamlanivimab and etesevimab, which are expected to
overcome or prevent antibody resistance.
REFERENCES
Abès R, Teillaud JL. Modulation of tumor immunity by
therapeutic monoclonal antibodies. Cancer Metastasis
Rev. 2011;30(1):111-124.
Anti-SARS-CoV-2 neutralizing monoclonal antibodies:
clinical pipeline - PubMed. Accessed December 29,
2021.
Bayer V. An Overview of Monoclonal Antibodies. Semin
Oncol Nurs. 2019;35(5):150927.
Cruz-Teran C, Tiruthani K, McSweeney M, Ma A, Pickles
R, Lai SK. Challenges and opportunities for antiviral
monoclonal antibodies as COVID-19 therapy. Adv
Drug Deliv Rev. 2021;169:100-117.
Chau, C. H., Steeg, P. S., & Figg, W. D. (2019). Antibody–
drug conjugates for cancer. The Lancet, 394(10200),
793–804.
Chen, X., Li, R., Pan, Z., Qian, C., Yang, Y., You, R., Zhao,
J., Liu, P., Gao, L., Li, Z., Huang, Q., Xu, L., Tang, J.,
Tian, Q., Yao, W., Hu, L., Yan, X., Zhou, X., Wu,
Y., … Ye, L. (2020). Human monoclonal antibodies
block the binding of SARS-CoV-2 spike protein to
angiotensin converting enzyme 2 receptor. Cellular &
Molecular Immunology, 17(6), 647–649
Cohen, M. S. (2021). Monoclonal Antibodies to Disrupt
Progression of Early Covid-19 Infection. The New
England Journal of Medicine, 384(3), 289–291
Crowe, J. E., Firestone, C. Y., & Murphy, B. R. (2001).
Passively acquired antibodies suppress humoral but not
cell-mediated immunity in mice immunized with live
attenuated respiratory syncytial virus vaccines. Journal
of Immunology (Baltimore, Md.: 1950), 167(7), 3910–
3918
Dees, S., Ganesan, R., Singh, S., & Grewal, I. S. (2021).
Bispecific Antibodies for Triple Negative Breast
Cancer. Trends in Cancer, 7(2), 162–173.
Jahanshahlu L, Rezaei N. Monoclonal antibody as a
potential anti-COVID-19. Biomed Pharmacother
Biomedecine Pharmacother. 2020;129:110337.
Marabelle A, Gray J. Tumor-targeted and immune-targeted
monoclonal antibodies: Going from passive to active
immunotherapy. Pediatr Blood Cancer.
2015;62(8):1317-1325.
Marovich, M., Mascola, J. R., & Cohen, M. S. (2020).
Monoclonal Antibodies for Prevention and Treatment
of COVID-19. JAMA, 324(2), 131–132
Scott AM, Wolchok JD, Old LJ. Antibody therapy of
cancer. Nat Rev Cancer. 2012;12(4):278-287.
Taylor, P. C., Adams, A. C., Hufford, M. M., de la Torre,
I., Winthrop, K., & Gottlieb, R. L. (2021). Neutralizing
monoclonal antibodies for treatment of COVID-19.
Nature Reviews Immunology, 21(6), 382–393
Wrapp, D., Wang, N., Corbett, K. S., Goldsmith, J. A.,
Hsieh, C.-L., Abiona, O., Graham, B. S., & McLellan,
Therapeutic Monoclonal Antibodies: Clinical Applications
341

J. S. (2020). Cryo-EM structure of the 2019-nCoV
spike in the prefusion conformation. Science
Zhou, P., Yang, X.-L., Wang, X.-G., Hu, B., Zhang, L.,
Zhang, W., Si, H.-R., Zhu, Y., Li, B., Huang, C.-L.,
Chen, H.-D., Chen, J., Luo, Y., Guo, H., Jiang, R.-D.,
Liu, M.-Q., Chen, Y., Shen, X.-R., Wang, X., … Shi,
Z.-L. (2020). A pneumonia outbreak associated with a
new coronavirus of probable bat origin. Nature,
579(7798), 270–273
ICBB 2022 - International Conference on Biotechnology and Biomedicine
342
