
Mechanisms of Resistance to Cancer Immunotherapy
Sitong Zhao
*
Department of Biochemical Engineering, INSA, Université de Toulouse, Toulouse 31013, France
Keywords: T Cells, Immune Checkpoint Blockade, Tumor Microenvironment, Gut Microbiome, Immunotherapy,
Resistance Mechanisms.
Abstract: Cancer immunotherapy has taken center stage in recent years as it can elicit a long-lasting anti-cancer
response. However, the response rates are not consistently high in every patient. The majority of cancers still
develop resistance to immunotherapy ranging from tumor intrinsic, microenvironment associated or host-
related pathways. These include aberrant neoantigen presentation/processing, over-activation of tumor-cell-
intrinsic signaling pathways, aberrant epigenetic regulation, presence of immunosuppressive cells, cytokines
and chemokines, overexpression of multiple immune checkpoints and composition of gut bacteria. This
review will focus on understanding the resistance mechanisms to immunotherapy in cancer and discuss ways
to overcome this.
1 INTRODUCTION
Cancer immunotherapy utilizes or reactivates the
patient's immune system, especially their T cells, to
kill cancer cells. Meanwhile, it exerts a long-lasting
anti-cancer response on the human body, which is
unmatched compared to other therapies (Ribas &
Wolchok, 2018). As evidence shows, blocking PD-
1/PD-L1 or CTLA-4 pathways can induce long-
lasting remission of cancers including melanoma,
urothelial cancer, head and neck squamous cell
carcinoma (HNSCC), lung cancer and renal cell
carcinoma (RCC). These therapies have also obtained
FDA approvals (Gong et al., 2018). Additional to the
PD-1/PD-L1/CTLA-4 immunotherapies, several
other targeted immunotherapy options range from
chimeric antigen receptor-modified T cells (CAR-T
cell) therapy, cancer vaccines, and oncolytic viruses,
which have shown positive results clinically and pre-
clinically. For example, the CAR-T cell therapy for B
cell malignancies has been proven effective and safe,
especially one of which targeting CD19 reached an
overall survival rate of 78% (Maude et al., 2014).
Although cancer immunotherapy has been widely
and rapidly applied to treat various types of cancer,
only a few patients (responders) have benefited from
them due to the complexity of immune systems.
Many patients do not produce any clinical benefit
(non-responders/ innate resistance) after
immunotherapy. For example, tumors that have
limited T cells in the tumor microenvironment
(TME), called immune cold or immune-desert
cancers such as prostate cancer, have had minimal
benefit from immunotherapy (Galon & Bruni, 2019;
Hegde et al., 2016). This form of innate resistance can
also be seen in glioblastoma and breast cancer, which
have low objective response rates with the anti- PD-
1/PD-L1 therapy (Dirix et al., 2018; Hansen et al.,
2018; Lukas et al., 2018). Additionally, some patients
first respond to immunotherapy but subsequently
develop acquired resistance (Jenkins et al., 2018).
Therefore, it is vitally important to determine the
mechanism through which cancers regulate
immunotherapy resistance.
The resistance mechanisms to cancer
immunotherapy are divided into three parts, tumor-
cell-intrinsic mechanism, TME-related mechanism,
and host-related mechanism. The tumor-cell-intrinsic
mechanism includes the alteration of neoantigen,
tumor cell signaling pathway and epigenetic
regulation. The TME-related mechanism involves
immunosuppressive cells, cytokines, chemokines and
multiple immune regulators. Finally, the host-related
mechanism is associated with patients' gender, age
and gut bacteria. This review will concentrate on the
mechanisms of resistance to cancer immunotherapy
352
Zhao, S.
Mechanisms of Resistance to Cancer Immunotherapy.
DOI: 10.5220/0012021000003633
In Proceedings of the 4th International Conference on Biotechnology and Biomedicine (ICBB 2022), pages 352-369
ISBN: 978-989-758-637-8
Copyright
c
2023 by SCITEPRESS – Science and Technology Publications, Lda. Under CC license (CC BY-NC-ND 4.0)

from these three parts above and discuss ways to
overcome immunotherapy resistance.
2 TUMOR-CELL INTRINSIC
MECHANISM
2.1 Alteration of Neoantigen
Though cancers have been known to be infiltrated by
antigen-specific CD8+ cytotoxic immune cells, they
often do not elicit an immune response. The ability of
cancers to initiate an adaptive immune response
depends on the presentation of neoantigenic peptides
by the cancer cell and their subsequent recognition by
cytotoxic cells (Reeves & James, 2017; Snyder et al.,
2014). A high tumor mutational burden (TMB),
denoted by increased mutations within a cancer cell,
usually accompanies the production of increased
neoantigens by cancer (Hugo et al., 2017; Perumal et
al., 2020; Rizvi et al., 2015; Schumacher & Schreiber,
2015). This indicates higher immunogenicity within
the tumor and immunotherapy targeting these tumors
have produced a favorable response. However,
cancers have the ability to alter the expression of and
presentation of neoantigens to aid in immune escape
(Veldman et al., 2020) (Figure 1).
High TMB cancers such as non-small cell lung
cancer (NSCLC) and melanoma produce increased
neoantigens, resulting in a favorable response to anti-
PD-1/PD-L1 therapy (Goodman et al., 2017b). By
contrast, tumors such as prostate cancer with low
TMB, which lack neoantigens, are less sensitive to
immunotherapy (Schumacher & Schreiber, 2015).
Additionally, Hellmann et al. found that high TMB
positively correlated to an immunotherapy response,
durable benefit, and progression-free survival with
immune checkpoint blockade treatment (Hellmann et
al., 2018). However, several studies have pointed out
that the clinical efficacy of immunotherapy does not
strongly depend on high TMB alone (Goodman et al.,
2017a; Gromeier et al., 2021; Strickler et al., 2021).
For example, patients with glioblastoma, a low TMB
cancer, had favorable survival responses to
immunotherapy (Gromeier et al., 2021).
Studies have also found that epigenetic changes
resulting in loss of neoantigen expression can aid
cancers to evade immune surveillance (De Vries et
al., 1997). For example, hypermethylation of the
promoter of neoantigen expressing genes during
transcription resulted in neoantigen silencing
(Rosenthal et al., 2020).
Abnormal antigen processing can lead to errors in
antigen presentation, impairing cytotoxic CD8+ T
cells to recognize the antigen, leading to immune
escape. In general, neoantigenic peptides are
processed into antigenic peptides by the proteasome
together with low molecular mass proteins (LMP).
Processed peptides are transported into the
endoplasmic reticulum (ER) via the transporter
associated with antigen processing proteins (TAP)
and assembled with human leukocyte antigen (HLA)
and Beta-2-Microglobulin (B2M) to form the major
histocompatibility complex class 1 (MHC-1). These
complexes are then transported on the tumor cell
surface, which aids in T cell recognition (SELIGER
et al., 1997). Naturally, all of these steps are
indispensable for the presentation of neoantigens. In
cancer patients, loss and down-regulation of LMP2,
LMP7 and TAP1 were reported, resulting in the
failure of presentation of neoantigens (Meissner et al.,
2005). The expression rates of LMP2, LMP7, TAP1,
TAP2, and HLA were also found to predict the overall
survival of cancer immunotherapy (Meissner et al.,
2005). Studies have found that loss of heterozygosity
and loss of function of the HLA gene, which is one of
the mechanisms causing immune evasion
(Mcgranahan et al., 2017). After treatment with an
RNA mutanome vaccine, B2M-deficient patients
with melanoma lacking presentation of neoantigens
had increased tumor growth, leading to recurrence,
indicating that this is also one of the resistance
mechanisms to immunotherapy (Sahin et al., 2017).
Tumor cell division can produce new mutant
subclones leading to intratumoral heterogeneity. The
selective pressure by immune sculpting can result in
the expansion of subclones that lack neoantigens or
down-regulate their genes in presentation machinery,
aiding cancer growth and reducing the effect of
immunotherapy (Mcgranahan et al., 2016).
Mechanisms of Resistance to Cancer Immunotherapy
353
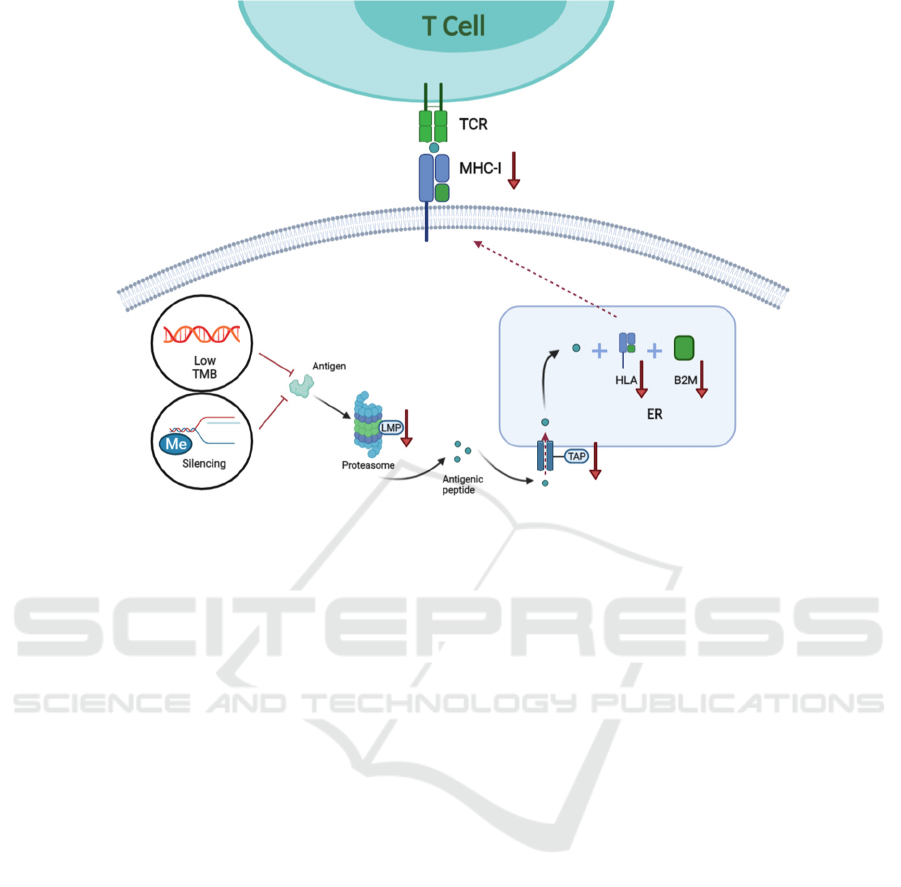
Figure 1: Neoantigen-related mechanism of resistance to immunotherapy.
Tumor mutation generates tumor-specific
peptides or neo-antigenic peptides. Peptides produced
are processed into antigenic peptides by the
proteasome where the LMP is involved. Next, the
antigenic peptides are transported into the ER, where
they are loaded onto the MHC class 1 complex which
consists of HLA-A, B, C and B2M. Finally, MHC is
transported and displayed on the tumor cell surface
and recognized by the TCR of T cells. Lack of
neoantigen due to low TMB, loss of neoantigen due
to the silencing of neoantigen (such as methylation)
and disruption in antigen presentation machinery
(such as the abnormal expression of LMP, TAP, HLA
and B2M) can result in immunotherapy resistance.
2.2 Epigenetic Regulation of Antigen
Presentation and Recognition
Pathway
Aberrant epigenetic regulation has been reported to
mediate various tumor-related genes, induce tumor
progression and metastasis and promote
immunotherapy resistance (Baylin & Jones, 2016;
Maio et al., 2015). Epigenetic regulation can also
suppress the expression and presentation of tumor-
associated antigens through processes like DNA
methylation, acetylation and histone modification
(Cao & Yan, 2020). In tumors resistant to
immunotherapy, down-regulation of immunogenic
antigens through DNA methylation has been reported
and DNA demethylating agents could restore the
expression of neoantigens (Wylie et al., 2019). As
mentioned above, HLA, TAP and B2M are involved
in the antigen presentation machinery. The promoter
region of the HLA gene has been reported to be
methylated, inhibiting its expression and resulting in
an impaired CD8+ T cell response (Luo et al., 2018).
Methylation was also observed in the promoter region
of B2M (Snahnicanova et al., 2020). Similarly,
reduced recruitment of histone acetyltransferase to
TAP-1 promoter regions was linked to its reduced
transcription, leading to dysfunctional antigen
presentation (Setiadi et al., 2007). This imbalance in
proteins involved in antigen presentation via
epigenetic regulations can result in a lack of
immunogenic antigens, resulting in resistance to
immunotherapy.
The function of T cells is also regulated by
epigenetic modification. For instance, PD-1, CTLA-
4, lymphocyte-activation gene 3 (LAG-3), and T cell
immunoglobulin domain and mucin domain 3 (TIM-
3), were demethylated or hypomethylated leading to
T-cell exhaustion (Sasidharan Nair et al., 2018).
Furthermore, the enhancer of zeste homologue 2
(EZH2) induced H3 trimethylation and DNA
methyltransferase 1 (DNMT1) induced DNA
methylation, leading to a decrease in the expression
of chemokines CXCL9 and CXCL10, important for T
ICBB 2022 - International Conference on Biotechnology and Biomedicine
354

helper cell recruitment into the tumor stroma
(Nagarsheth et al., 2016; D. Peng et al., 2015).
Cancer cells also have the ability to prevent T cell-
induced apoptosis by regulating cell-extrinsic
apoptotic pathways through epigenetic regulation.
The binding of Fas-L on the CD8+ lymphocytes with
its counterpart FAS on the tumor cell can trigger
apoptosis of the cancer cell. However, cancers can
downregulate the expression of Fas and TRAIL-R1
proteins by DNA methylation, preventing CD8+
induced apoptosis (Hopkins-Donaldson et al., 2003).
Similarly, histone deacetylation has also been
implicated in silencing TRAIL and Fas signaling
pathways (Insinga et al., 2005).
Epigenetic induced immunotherapy resistance
can be overcome by combining immunotherapy with
drugs targeting epigenetic modifications, and this
combination has produced favorable outcomes in
recent clinical trials (Gallagher et al., 2017; Pauken et
al., 2016). For example, histone deacetylase inhibitor
(HDACi) can overcome the resistance to monoclonal
anti-CD52 antibody (alemtuzumab) in patients with
T-cell prolymphocytic leukemia (Hasanali et al.,
2015). Likewise, DNA hypomethylating agents
(DHAs) guadecitabine combined with anti-CTLA-4
antibody ipilimumab displayed promising tumor
immunomodulation through the upregulation of HLA
class I and increasing CD8+, PD-1+ T cells and
CD20+ B cells in a phase Ib trial (Di Giacomo et al.,
2019).
2.3 Tumor-Intrinsic Cell Signaling
Pathway
Studies have shown that the alteration of various
oncogenic cell signaling pathways can affect the
immune response to tumors (Aldea et al., 2021;
Kalbasi & Ribas, 2020). The changes may affect the
expression of transcription factors, resulting in
aberrant antigen presentation, expression of immune
checkpoint-related proteins, or the production of
immunosuppressive cytokines (Fares et al., 2019; W.
Peng et al., 2016). It is also vital to note that the role
of the tumor cell signaling pathway will have a
context-dependent role in promoting
immunosuppression.
2.3.1 Alteration of PI3k/AKT Signaling
Pathway
The PI3K/AKT signaling network is activated via
ligand binding to receptor tyrosine kinases (RTK), G
protein-coupled receptors (GPCR), or cytokine
receptors. This pathway plays a central role in
promoting cell survival and growth (Hoxhaj &
Manning, 2020). The proteins phosphatidyl-inositol-
3-kinases (PI3Ks), protein kinase B (AKT),
mammalian target of rapamycin (mTOR) and
phosphatase and tensin homolog (PTEN) are
involved in this signaling network. PTEN is the
negative regulator of PI3k/AKT signaling netwpork,
which inhibits PI3K and prevents AKT activation
(Porta et al., 2014). Thus, loss of PTEN induces
abnormally activation of the PI3k/AKT signaling
pathway (Porta et al., 2014).
The expression of PD-L1, one of the immune
checkpoint inhibitory receptors, has been reported to
be controlled by the PI3K-AKT-mTOR pathway.
Membranous expression of PD-L1 is positively
correlated with the activation of mTOR, thereby
reducing tumor-infiltrating T cells, increasing
regulatory T cells (Tregs), and resulting in immune
escape (Lastwika et al., 2016). Loss of PTEN has
been shown to up-regulate immunosuppressive
cytokines of CCL2 and VEGF, which can recruit
tumor-associated macrophages (TAMs) and aid in
immune escape (W. Peng et al., 2016; Voron et al.,
2014; H. Yang et al., 2020). Additionally, increased
VEGF can also cause abnormal angiogenesis. This
potentiates the immunosuppressive phenotype by
recruiting suppressive immune cells, such as Tregs
and myeloid-derived suppressor cells (MDSCs),
reported to promote resistance to immunotherapy in
melanoma (W. Peng et al., 2016; Voron et al., 2014).
Mutations in PIK3CA coding the p110 subunit of
PI3K cause abnormal activation of the PI3K and can
reduce immune infiltration (Borcoman et al., 2019;
Madsen et al., 2018). Direct activation of the PI3k-
AKT pathway through activating mutations can also
lead to a suppressive tumor microenvironment. For
example, RHOA mutations in gastric cancer (GC) can
trigger the PI3K-AKT-mTOR pathway to increase
the synthesis of free fatty acids (released by tumor
cells), which promotes Treg cell metabolism,
ultimately contributing to an increased Tregs in TME
(Kumagai et al., 2020).
2.3.2 Alteration of ERK/MAPK Signaling
Pathway
Similar to PI3K/AKT signaling pathway, mitogen-
activated protein kinase (MAPK) signaling is
activated when receptors such as RTKs and GPCRs
bind to their ligand. Hyperactivation of this pathway
results in cancer development and progression. The
main proteins involved in this signaling pathway are
RAS, RAF, MEK and ERK from upstream to
downstream (Guo et al., 2020).
Mechanisms of Resistance to Cancer Immunotherapy
355

The expression of PD-L1 can be regulated by
ERK/MAPK signaling pathway, and the activation of
this pathway can also decrease the TILs (Loi et al.,
2016; Sumimoto et al., 2016). Abnormal EGFR
signaling via the MAPK pathway can up-regulate the
expression of PD-L1 through the p-ERK1/2/p-c-Jun
transcription factors, causing T cell apoptosis (Chen
et al., 2015). The activation of RAS and its
downstream signaling MEK can also indirectly
promote PD-L1 expression by inhibiting AU-rich
element-binding protein tristetraprolin (TTP) by
kinase MK2 (Coelho et al., 2017). KRAS (one of RAS
family members) mutation mediates the inhibition of
interferon regulatory factor 2 (IRF2), leading to high
expression of CXCL3 to form an immunosuppressive
microenvironment (Liao et al., 2019). Furthermore,
the eukaryotic translation initiation complex (eIF4F)
located downstream of the PI3K-AKT and ERK-
MAPK pathway regulates PD-L1 transcriptional
factor STAT1, whose activation has a positive
correlation with the activation of PD-L1 (Cerezo et
al., 2018). Clinically, in patients who developed
hyperprogressive disease following anti-PD-1
therapy, it was found to have over activation of the
ERK/MAPK, PI3K/AKT, IGF-1 and TGF-b
signaling pathways, suggesting their role in
promoting immunotherapy acquired resistance
(Xiong et al., 2018).
2.3.3 Alteration of Wnt/β-Catenin Signaling
Pathway
Wnt/β-Catenin signaling pathway is activated upon
Wnt ligand binding to Frizzled receptors. As a result,
β-Catenin translocates into the nucleus and binds to
transcription factors of target genes related to cancer
progression (Zhang & Wang, 2020).
Studies have shown that the Wnt/β-catenin
pathway may promote immunotherapy resistance by
producing immunosuppressive cytokine IL-10 in
melanoma (Yaguchi et al., 2012). Melanoma cells
expressing β-catenin cannot produce C-C motif
chemokine ligand (CCL4), causing defective
recruitment of antigen-presenting CD103+ Dendritic
Cells (DCs). This can lead to the loss of the
chemokines derived by CD103+ DCs, such as CXC
motif chemokine ligand 9 (CXCL9) and CXCL10,
which reduce cytotoxic T lymphocytes (CTLs) tumor
infiltration and damage the anti-tumor immune
response (Spranger et al., 2015). Another example
also reported that in non-T-cell-inflamed tumors, the
activation of tumor-intrinsic WNT/β-catenin
signaling reduced immune cell infiltration (Luke et
al., 2019).
2.3.4 Alteration of Cell Signaling Pathways
Related to IFN
Tumor intrinsic interferon (IFN) signaling pathway is
activated via autocrine and paracrine IFNs binding
onto its receptor (IFNGR1/IFNGR2) (Dunn et al.,
2006; Reisländer et al., 2019). This mediates the
transcription of interferon-stimulated genes through
the JAK-STAT pathway, resulting in enhanced T cell
response and the tumor cells' apoptosis (Ni & Lu,
2018; Reisländer et al., 2019). However, contrary to
this, the IFN pathway has also been implicated in
resistance to immunotherapy.
One mechanism of acquired resistance to anti-PD-
1 immunotherapy has been linked to the mutation of
JAK1/2. This mutation prevents activation of
downstream IFN transcription factor, which impairs
the transcription of interferon receptors, resulting in
the lack of IFN receptors and decreasing the effect of
IFN (Zaretsky et al., 2016). JAK1/2 loss of function
mutation has also been shown to inhibit the
expression of PD-L1 and the response to anti-PD-L1
therapy (Shin et al., 2017). Mutations of IFN-γ
pathway genes, such as IFNGR1, IFNGR2, IRF-1 and
JAK2, also resulted in unfavorable responses to anti-
CTLA-4 therapy, which could be a mechanism of
resistance to immunotherapy (Gao et al., 2016).
Interestingly, anti-CTLA-4 therapy can increase the
interferon-γ response genes, including CTLA-4
through the JAK-STAT pathway, resulting in
resistance to CTLA-4 (Mo et al., 2018). It is also
shown that IFN-γ promoted PD-L1 expression
resulting in immune evasion via the cell signaling
pathway of JAK-STAT -IRF1 (Garcia-Diaz et al.,
2017).
3 TUMOR-CELL EXTRINSIC
MECHANISM
3.1 Immune Contexture of the Tumor
Microenvironment
In addition to tumor-intrinsic resistance mechanisms,
tumor TME also plays a vital role in causing
resistance to cancer immunotherapy. The TME
includes immunosuppressive cells, cytokines and
chemokines, which could impair cytotoxic cells and
the immune system (Galon & Bruni, 2019; Hegde et
al., 2016). Additionally, some tumors have been
reported to reduce the presence of T cells in the
microenvironment, such as prostate cancer (Galon &
Bruni, 2019; Hegde et al., 2016). The immune cell
ICBB 2022 - International Conference on Biotechnology and Biomedicine
356

composition within the tumor is also an essential
factor that could predict response to immunotherapy.
The TME consists of cytotoxic cells such as CD8+
T cells, NK cells and DC cells that aid immune
surveillance and cancer destruction. However, it also
contains MDSCs, Tregs and M2 macrophages, which
are immunosuppressive and induce immune system
dysfunction and tumor immune escape, resulting in
resistance to cancer immunotherapy (Aldea et al.,
2021). In the TME, MDSCs are able to inhibit the
proliferation and activity of CD8+ T cells and release
immunosuppressive cytokines, promoting tumor
proliferation and metastasis (Hou et al., 2020; Law et
al., 2020). Tregs are immunosuppressive to aid
immune homeostasis, but in tumors, they promote
tumor progression as a suppressor of anti-tumor
immunity via releasing immunosuppressive
cytokines and binding to tumor cells or antigen-
presenting cells (APCs), leading to T-cell exhaustion
(Takeuchi & Nishikawa, 2016). Unlike the above
two, Macrophages have a high degree of plasticity,
which have been classified as M1 and M2 (Mosser &
Edwards, 2008). M1 macrophages are involved in
anti-tumor immunity, while M2 macrophages (also
known as tumor-associated macrophages, TAMs) are
related to the progression and metastasis of tumors
and the formation of immunosuppressive TME
(Italiani & Boraschi, 2014). And its
immunosuppressive function is similar to MDSCs
(Italiani & Boraschi, 2014).
In the clinic, MDSCs, Tregs and TAMs frequency
were shown to be related to unfavorable prognosis
and shorter overall survival (OS) in various types of
cancer (Ai et al., 2018; Fridman et al., 2012). For
example, patients developing resistance to anti-PD-1
therapy showed an increased expression of TIM-3
binding to galectin-9 on MDSCs when the treatment
failed (Limagne et al., 2019).
Recently, there has been an influx in treatments
targeting the composition of the immune
microenvironment to prevent innate resistance to
immunotherapy. Combination treatment with CTLA-
4 and PD-L1 inhibitors has been reported to increase
T cell infiltration in immune cold prostate cancer
(Sharma et al., 2020). As mentioned above, TAMs
can also promote tumor angiogenesis. Treatment with
bispecific anti-ANG2/VEGF-A antibody (CrossMab,
A2V) successfully improved the survival rate of
vasculature-aberrant glioblastoma, owing to the
reprogramming of TAMs from M2 to M1 (Kloepper
et al., 2016). With the treatment of CD25-blocking
monoclonal antibody daclizumab, Tregs lost the
immunosuppressive function and restored the ability
to generate interferon-γ (Rech et al., 2012).
3.2 Expression of Immune-Modulatory
Factors
MDSCs, TAMs and Tregs are able to release
cytokines within the TME, promoting tumor immune
evasion (Haist et al., 2021). MDSCs can secrete
interleukin-10 (IL-10), IL-17 and transforming
growth factor-β (TGF-β). TAMs can also secrete
TGF-β and IL-10. This results in suppressing CD8+
T-cell function and promotes the immunosuppressive
function of Tregs (Huang et al., 2006; Wang et al.,
2019; Z. Yang et al., 2010). The proliferation and the
function of T cells can be inhibited by Tregs via
secreting cytokines, such as IL-35, IL-10 and TGF-β
(Jarnicki et al., 2006; Turnis et al., 2016). IL-35 can
also promote multiple inhibitory receptors such as
PD1, TIM-3, LAG-3 and cause T cell exhaustion
(Turnis et al., 2016). Besides T cells, NK cells are also
inhibited by the MDSCs secreting TGF-β1 (Li et al.,
2009). TGF-β1, secreted by MDSCs, TAMs and
Tregs, can also contribute to adaptive immunotherapy
resistance to anti-PD1 therapy by restricting T cell
infiltration (Mariathasan et al., 2018). Treatments co-
targeting these chemokines with immunotherapy
agents can prevent resistance and have shown clinical
benefits. A combination of anti-CXCR2 (CXCR2
expressing by MDSCs) with anti-PD1 was shown to
reduce tumor size and enhance T-cell infiltration
(Najjar et al., 2017). The bifunctional agent (anti-PD-
L1 and anti-TGFβ), Bintrafusp Alfa, enhanced tumor
cell lysis and reduced Tregs activity (Lind et al.,
2020).
3.3 Multiple Inhibitory Regulators
Immune checkpoints can regulate the activation of
CD8+ T cells, of which the most common ones are
PD-1 and CTLA-4. In addition to the two, there are
more inhibitory regulators which can bind to the
surface of tumor cells or APCs, leading to T-cell
exhaustion. These include LAG-3, TIM-3, T cell
immunoreceptor with Ig and ITIM domain (TIGIT),
and V-type immunoglobulin domain-containing
suppressor of T cell activation (VISTA) (Ding et al.,
2020; Kurachi, 2019). Immune checkpoint treatments
have shown to up-regulate secondary immune
regulators that cause immunotherapy resistance
(Nowicki et al., 2018). Therefore, the combination
treatment with multiple immune regulators can
enhance the effect of checkpoint blockade
monotherapy (Long et al., 2018; Seidel et al., 2018).
For example, the patients that showed no response to
monotherapy of ipilimumab (anti-CTLA-4) benefited
from a subsequent therapy of nivolumab (anti-PD-1)
Mechanisms of Resistance to Cancer Immunotherapy
357

in melanoma (Weber et al., 2013). There was also the
case when combining an anti-PD-L1 agent with an
anti-Tim-3 agent. Combination treatment reversed T
cell exhaustion and reduced the tumor growth in
colon carcinoma (Sakuishi et al., 2010). Combining
multiple checkpoint inhibitors could be a promising
strategy to overcome immunotherapy resistance.
3.4 Tumor Cell-Extrinsic Metabolic
Pathway
The metabolic pathway around the TME also plays a
vital role in promoting resistance to immunotherapy.
It includes the pathway of glycolysis, the pathway of
depleting various amino acids, and the production of
adenosine. Cancer cells and surrounding immune
cells undergo these metabolic reprogramming that
can promote resistance to immunotherapy (Fares et
al., 2019).
In 1926, Warburg proposed that tumor cells obtain
energy through tumor-specific glycolysis, producing
a large amount of lactic acid (Warburg, 1956;
Warburg et al., 1927). Aberrant glycolysis results in
the production of lactate and H
+
, which are released
by various H
+
transporters (such as monocarboxylate
transporter 4, MCT4) into the extracellular matrix.
This results in a lower extracellular pH (pH
e
) and
tumor acidosis (Corbet & Feron, 2017). Warburg
effect in cancer cells can influence the immune
response of immunotherapy partly via glucose
competition, lactate production, and the creation of an
acidic TME.
Studies have shown a glucose competition
between tumor cells and T cells, which restricts T cell
function by reducing their glycolytic capacity,
cytolytic activity, cytokines production and IFN-γ
production. This leads to T cell hyporesponsiveness
and an impaired immune response (Cham et al., 2008;
Chang et al., 2015). However, recent findings have
suggested that MDSCs and TAMs have the highest
glycolytic capacity, outcompeting the T cells for
glucose over the cancer cells (Reinfeld et al., 2021).
The resulting lactate produced from glycolysis was
also shown to decrease the cytotoxic activity and the
expression of granzyme and perforin in NK cells,
suppressing their anti-tumor immune response
(Husain et al., 2013). Additionally, a lower pH
e
in
TME causes the reduction of cytotoxic cytokines,
such as tumor necrosis factor-alpha (TNF-α) and
interferon-gamma (INF-γ) (Müller et al., 2000). This
acidic condition has also been reported to promote
macrophage polarization towards an
immunosuppressive phenotype, TAM (Bohn et al.,
2018).
Within the TME, there is also competition for the
consumption of amino acids, such as tryptophan,
cysteine, and arginine by the immunosuppressive
cells. MDSCs can reduce the level of local tryptophan
by expressing indoleamine 2,3 dioxygenase (IDO),
causing the reduction of nutrients for T cells leading
to their dysfunction (Yu et al., 2013). In murine
models, the combination therapy of checkpoint
inhibitors with IDO blockade can reactivate these
dysfunctional T cells and restore their IL-2
production (Spranger et al., 2014). Additionally,
MDSCs are also known to sequester essential amino
acid cystine and reduce the availability of cysteine
within the TME. Reduction in free cysteine can
downregulate T cell activation as T cells cannot
produce cysteine due to lack of cystathionase
(GMÜNDER et al., 1991; Srivastava et al., 2010). L-
arginine, which regulates the T-cell cycle
progression, can also be depleted by MDSCs and
TAMs through arginase I, further contributing to
immunotherapy resistance (Barbul & Dawson, 2018;
Munder et al., 2006; Rodriguez et al., 2007).
CD39 and CD73 are known to convert free ATP
into adenosine which is released into the TME and
can inhibit T cell function via its interaction with A2A
receptors (A2AR) or A2B receptors (A2BR) on
immune cells. This results in immune suppression via
the increased expression of various immune
checkpoints and decreased cytokine production (B.
Allard et al., 2017; D. Allard et al., 2017; Cekic &
Linden, 2016; Sek et al., 2018). Extracellular
adenosine can promote CTLA-4 expression in Tregs
and reduce IL-7 levels, which aids in the development
and survival of naïve T cells (Cekic et al., 2013;
Deaglio et al., 2007). The activation of A2BR can also
promote the expansion of MDSCs (Ryzhov et al.,
2011). Preclinically, it has shown that combinations
of checkpoint inhibitors with A2AR blockade can
inhibit adenosine and restore T cell function, and the
combination with CD73 blockade can reduce the
conversion of adenosine. Targeting the metabolic
pathways in cancer can be a potential therapeutic
strategy to overcome resistance to immunotherapy
(B. Allard et al., 2013; Beavis et al., 2015).
4 HOST-RELATED MECHANISM
OF RESISTANCE
Host-associated factors such as age, gender, and
composition of intestinal bacteria may also affect the
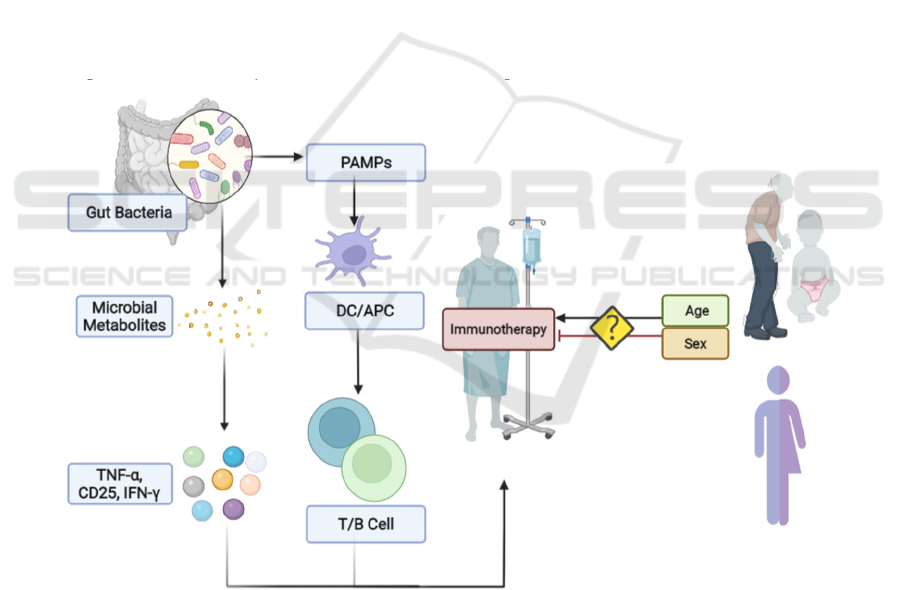
response to immunotherapy (Figure 2). It had been
previously reported that aging could dampen the
ICBB 2022 - International Conference on Biotechnology and Biomedicine
358
function of the immune system, thereby affecting the
efficacy of immunotherapy (Hong et al., 2019).
However, a recent meta-analysis indicated no
association between age and the effectiveness of
immunotherapy (F. Yang et al., 2020). Similarly,
several studies have also shown opposing results
regarding the association between the efficacy of
immunotherapy and gender (Wallis et al., 2019; F.
Yang et al., 2020).
There is growing evidence suggesting that the
host-microbiome has a role in modulating response to
cancer immunotherapy. It was reported that the
pathogen-associated molecular patterns (PAMPs)
from the intestinal microbiome, such as
lipopolysaccharide, could directly activate APCs
such as DCs, which can translocate into mesentery
lymph nodes (MLNs) to prime the B and T cells at
distant sites (Stary et al., 2015). Additionally, the gut
microbiomes are able to induce the secretion of
immunomodulatory factors to regulate the immune
system. For example, short-chain fatty acids (SCFAs)
(such as pentanoate and butyrate) are microbial
metabolites, which can increase cytokine production,
such as TNF-α, CD25 and IFN-γ to enhance the anti-
tumor function of T cells and CAR-T therapy (Luu et
al., 2021). In germ-free (GF) mice, tumor progression
was not controlled by anti-CTLA-4 therapy, while the
GF mice fed with B. uniformis restored the
responsiveness to anti-CTLA-4 therapy (Vétizou et
al., 2015). As a result, the combination of
immunotherapy and gut microbiome seems to be a
promising strategy to overcome the resistance in the
clinic. The treatment with Bifidobacterium can
enhance anti-PD-L1 therapy in vivo, resulting in
abolishing the growth of tumors via increasing
expression of the genes involved in CD8+ T cell
activation, antigen processing and presentation, and
interferon signaling (Sivan et al., 2015). Furthermore,
a higher microbial diversity with Bifidobacterium
longum, Collinsella aerofaciens, Enterococcus
faecium, Ruminococcaceae, Clostridiales, and
Faecalibacterium is also associated with a better
prognosis for checkpoint blockade therapy
(Gopalakrishnan et al., 2018; Matson et al., 2018).
Figure 2: Host-related mechanism of resistance to cancer immunotherapy.
The association between age or gender and effects
on immunotherapy response is controversial. The
gut microbiome can activate PAMPs, which in turn
recruits APCs to prime T and B cells. The
composition of the gut microbiome is vital in
influencing immunotherapy response. Gut microbiota
has also been reported to up-regulate the production
of TNF-α, CD25 and IFN-γ, which can enhance the
effect of cancer immunotherapy.
Mechanisms of Resistance to Cancer Immunotherapy
359

5 DISCUSSION AND
CONCLUSION
In this review, we have identified multiple
mechanisms of immunotherapy resistance and
potential areas for future research. Resistance to
immunotherapy emerges from a complex list of
factors which are tumor-cell intrinsic, extrinsic
(Figure 3) or host-related. Research into patient
stratification for immunotherapy and identifying
resistance biomarkers are both essential in ensuring
better treatment responses. With the emergence of
various immunotherapy resistances, combination
treatments with other targeted therapies have
gradually entered the clinical stage. The main aim of
this combination treatment is to use targeted therapies
to block immunotherapy resistance mechanisms such
as tumor intrinsic and extrinsic pathways mentioned
in this review. The combination of CTLA-4 and PD-
1 blockers has achieved great success in the clinic,
lessening the resistance to the monotherapy in
multiple types of cancer (Rotte, 2019). Similarly,
indoximod was added to the treatment of
pembrolizumab to overcome the resistance from the
up-regulated expression of IDO (Zakharia et al.,
2021). Combination of DNA hypomethylating agent
(DHA) guadecitabine with anti-CTLA-4 antibody
ipilimumab resulted in increased CD8+ infiltration
and prevented resistance induced from HLA class I
downregulation (Di Giacomo et al., 2019). Likewise,
the pan-PI3K inhibitor copanlisib enhanced the effect
of the monotherapy of immune checkpoint inhibitors
(Yan et al., 2021). Identifying combination treatments
that can improve the primary immunotherapy
response and block immunotherapy resistance
mechanisms is an important strategy needed to
achieve better treatment results. Table 1 lists clinical
trials of current combination therapy programs.
Table 1: List of combination therapy against the resistance to cancer immunotherapy.
Resistance Mechanism
Clinical Trial
ID
Cancer Type Cancer Characterization Combination Therapy Agents
Targets
(respectively)
Phase
Neoantigen
Lack of
neoantigen
NCT03827044 Colon cancer Stage III 5-FU + Avelumab
Chemotherapy
and PD-L1
Phase 3
NCT04397003
Small cell lung
cancer
Extensive stage Neoantigen DNA vaccine + Durvalumab
Neoantigen and
PD-L1
Phase 2
NCT03867175 Lung cancer Metastatic or stage IV
Stereotactic Body Radiation +
Pembrolizumab
Radiation
therapy and PD-1
Phase 3
Alteration of cell
intrinsic signaling
pathway
PI3K/AKT
signaling
pathway
NCT03257722
Non-small cell
lung cancer
Metastasic or Recurrent Idelalisib + Pembrolizumab PI3K-δ and PD-1
Phase
1b/2
NCT03502733
Solid tumor or
lymphoma
Metastatic or Recurrent
or Unresectable or stage
III/ IV
Copanlisib + Ipilimumab + Nivolumab
PI3K and CTLA4
and PD-1
Phase 1b
NCT03190174
Sarcoma and
certain cancers
Advanced Nab-Rapamycin + Nivolumab mTOR and PD-1 Phase 1/2
ERK/MAPK
signaling
pathway
NCT01754376 Melanoma
Mutant in the BRAF
gene
Vemurafenib + Aldesleukin(IL-2)
BRAF and T
cells/NK cells
Phase 2
NCT04163237 Liver cancer Advanced Sorafenib + PD-1
ERK/MAPK
signaling
pathway + VEGFR
and PD-L1
Phase 3
NCT03363867
Ovarian &
Fallopian tube
cancer &
Peritoneal
carcinoma
Recurrent
Cobimetinib + Bevacizumab +
Atezolizumab
MEK and VEGF
and PD-L1
Phase 2
VEGF-related
signaling
pathway
NCT04715633
Colorectal
cancer
Microsatellite instability
high
Apatinib + Camrelizumab VEGFR2 and PD-1 Phase 2
NCT03517449
Endometrial
cancer
Advanced Lenvatinib + Pembrolizumab VEGFR and PD-1 Phase 3
NCT04356729 Melanoma
Stage III/ IV or
unresectable
Bevacizumab + Atezolizumab VEGF and PD-L1 Phase 2
NCT01950390 Melanoma
Stage III/ IV or
unresectable
Bevacizumab + Ipilimumab VEGF and CTLA4 Phase 2
HER-related
signaling
pathway
NCT04740918 Breast Cancer
Metastasic & HER-2+ &
PD-L1+
Trastuzumab + Atezolizumab HER-2 and PD-L1 Phase 3
NCT03082534
Head & Neck
Squamous Cell
Carcinoma
Metastasic or Recurrent Cetuximab + Pembrolizumab EGFR and PD-1 Phase 2
Epigenetic
regulation
Alteration of
epigenetic
regulation
NCT03765229 Melanoma - Entinostat + Pembrolizumab HDAC and PD-1 Phase 2
NCT02608437 Melanoma Metastasic SGI-110 + Ipilimumab
DNMT and CTLA-
4
Phase 1
ICBB 2022 - International Conference on Biotechnology and Biomedicine
360
Tumor
microenvironment
TAMs and
MDSCs
NCT02452424
Melanoma and
other solid
tumors
Advanced PLX3397 + Pembrolizumab CSF1R and PD-1 Phase 1/2
NCT02880371 Solid tumors Advanced ARRY-382 + Pembrolizumab CSF1R and PD-1
Phase
1b/2
Multiple
Inhibitory
Regulators
NCT03084471 Solid tumors Advanced Durvalumab + Tremelimumab PD-L1 and CTLA4 Phase 3
NCT03680508 Liver cancer
Primary or Advanced or
Unresectable adult
primary
Cobolimab + Dostarlimab TIM-3 and PD-1 Phase 2
NCT04370704 Melanoma Advanced
INCAGN02385 + INCAGN02390 +
INCMGA00012
LAG-3 and TIM-3
and PD-1
Phase 1/2
Lack of T/NK
Cells
NCT01629758 Solid tumors - Denenicokin(IL-21) + Nivolumab
T/NK cells and
PD-1
Phase 1
NCT02989714
Renal Cell
Carcinoma
Metastasic IL-2 + Nivolumab
T/NK cells and
PD-1
Phase 1/2
IDO NCT02752074 Melanoma
Unresectable or
metastatic
Epacadostat + Pembrolizumab IDO and PD-1 Phase 3
Host-related
Host
Microbiome
NCT04924374 Lung Cancer Advanced
Microbiota capsule +
Pembrolizumab/Nivolizumab/Atezolizumab
Gut microbiome
and PD-1
Not
Applicable
NCT03341143 Melanoma -
Fecal Microbiota Transplant +
Pembrolizumab
Gut microbiome
and PD-1
Phase 2
Immunotherapy treatment has been linked to
producing a durable anti-tumor response and using
combination therapy to prevent resistance
mechanisms can enhance this. Currently, new
strategies are being used to recruit patients to
immunotherapy trials, such as measuring their tumor-
infiltrating T cell counts, PD-L1 expression and MSI
status (Hegde & Chen, 2020). However, current
clinical practices still lack prediction biomarkers for
immunotherapy resistance. Research into better
companion diagnostic tools that can offer
personalized immunotherapy regimes or
combinations can provide a long-lasting response for
patients.
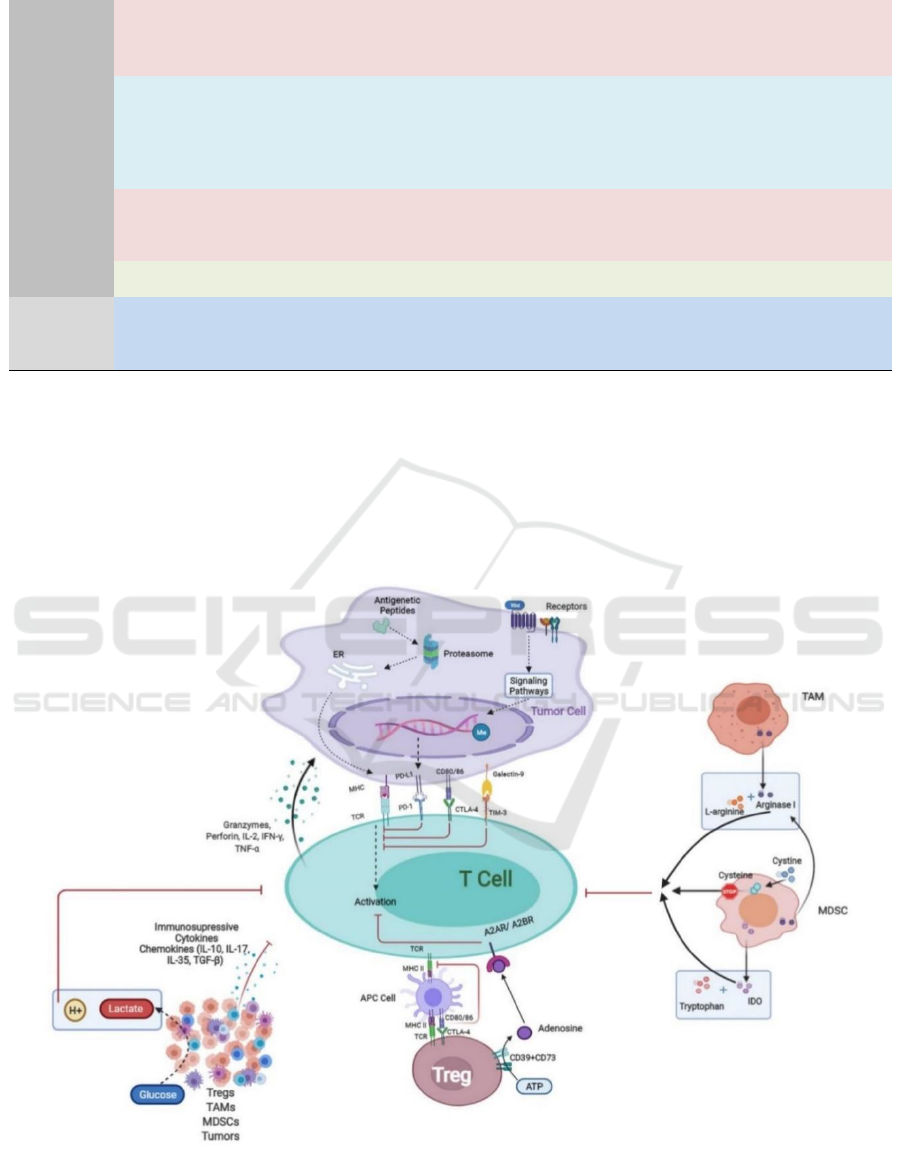
Figure 3: Tumor cell-intrinsic and TME-related mechanisms of resistance to cancer immunotherapy.
Mechanisms of Resistance to Cancer Immunotherapy
361

Tumor cell-intrinsic mechanism includes
resistance via aberrant neoantigen
presentation/processing, over activation of tumor-
cell-intrinsic signaling pathway and epigenetic
regulation. TME-related resistance mechanisms
include 1) increased infiltration of
immunosuppressive cells, 2) secretion of cytokines
and chemokines, 3) metabolism of glucose, amino
acids and adenosine, and 4) expression of multiple
inhibitory regulators, resulting in dysfunction of the
immune system.
REFERENCES
Ai, L., Mu, S., Wang, Y., Wang, H., Cai, L., Li, W., & Hu,
Y. (2018). Prognostic role of myeloid-derived
suppressor cells in cancers: a systematic review and
meta-analysis. BMC Cancer, 18(1), 1–9.
https://doi.org/10.1186/s12885-018-5086-y
Aldea, M., Andre, F., Marabelle, A., Dogan, S., Barlesi, F.,
& Soria, J. C. (2021). Overcoming resistance to tumor-
targeted and immune-targeted therapies. Cancer
Discovery, 11(4), 874–899.
https://doi.org/10.1158/2159-8290.CD-20-1638
Allard, B., Longhi, M. S., Robson, S. C., & Stagg, J.
(2017). The ectonucleotidases CD39 and CD73: Novel
checkpoint inhibitor targets. Immunological Reviews,
276(1), 121–144. https://doi.org/10.1111/imr.12528
Allard, B., Pommey, S., Smyth, M. J., & Stagg, J. (2013).
Targeting CD73 enhances the antitumor activity of
anti-PD-1 and anti-CTLA-4 mAbs. Clinical Cancer
Research, 19(20), 5626–5635.
https://doi.org/10.1158/1078-0432.CCR-13-0545
Allard, D., Turcotte, M., & Stagg, J. (2017). Targeting A2
adenosine receptors in cancer. Immunology and Cell
Biology, 95(4), 333–339.
https://doi.org/10.1038/icb.2017.8
Barbul, A., & Dawson, H. (2018). Arginine and immunity.
Diet Nutrition and Immunity, March, 199–216.
https://doi.org/10.1201/9781351071390
Baylin, S. B., & Jones, P. A. (2016). Epigenetic
determinants of cancer. Cold Spring Harbor
Perspectives in Biology, 8(9), 1–35.
https://doi.org/10.1101/cshperspect.a019505
Beavis, P. A., Milenkovski, N., Henderson, M. A., John, L.
B., Allard, B., Loi, S., Kershaw, M. H., Stagg, J., &
Darcy, P. K. (2015). Adenosine receptor 2A blockade
increases the efficacy of anti-PD-1 through enhanced
antitumor T-cell responses. Cancer Immunology
Research, 3(5), 506–517.
https://doi.org/10.1158/2326-6066.CIR-14-0211
Bohn, T., Rapp, S., Luther, N., Klein, M., Bruehl, T. J.,
Kojima, N., Aranda Lopez, P., Hahlbrock, J., Muth, S.,
Endo, S., Pektor, S., Brand, A., Renner, K., Popp, V.,
Gerlach, K., Vogel, D., Lueckel, C., Arnold-Schild, D.,
Pouyssegur, J., … Bopp, T. (2018). Tumor
immunoevasion via acidosis-dependent induction of
regulatory tumor-associated macrophages. Nature
Immunology, 19(12), 1319–1329.
https://doi.org/10.1038/s41590-018-0226-8
Borcoman, E., De La Rochere, P., Richer, W., Vacher, S.,
Chemlali, W., Krucker, C., Sirab, N., Radvanyi, F.,
Allory, Y., Pignot, G., Barry de Longchamps, N.,
Damotte, D., Meseure, D., Sedlik, C., Bieche, I., &
Piaggio, E. (2019). Inhibition of PI3K pathway
increases immune infiltrate in muscle-invasive bladder
cancer. OncoImmunology, 8(5), 1–17.
https://doi.org/10.1080/2162402X.2019.1581556
Cao, J., & Yan, Q. (2020). Cancer Epigenetics, Tumor
Immunity, and Immunotherapy. In Trends in Cancer
(Vol. 6, Issue 7, pp. 580–592). Cell Press.
https://doi.org/10.1016/j.trecan.2020.02.003
Cekic, C., & Linden, J. (2016). Purinergic regulation of the
immune system. Nature Reviews Immunology, 16(3),
177–192. https://doi.org/10.1038/nri.2016.4
Cekic, C., Sag, D., Day, Y. J., & Linden, J. (2013).
Extracellular adenosine regulates naive T cell
development and peripheral maintenance. Journal of
Experimental Medicine, 210(12), 2693–2706.
https://doi.org/10.1084/jem.20130249
Cerezo, M., Guemiri, R., Druillennec, S., Girault, I.,
Malka-Mahieu, H., Shen, S., Allard, D., Martineau, S.,
Welsch, C., Agoussi, S., Estrada, C., Adam, J.,
Libenciuc, C., Routier, E., Roy, S., Désaubry, L.,
Eggermont, A. M., Sonenberg, N., Scoazec, J. Y., …
Robert, C. (2018). Translational control of tumor
immune escape via the eIF4F–STAT1–PD-L1 axis in
melanoma. Nature Medicine, 24(12), 1877–1886.
https://doi.org/10.1038/s41591-018-0217-1
Cham, C. M., Driessens, G., O’Keefe, J. P., & Gajewski, T.
F. (2008). Glucose deprivation inhibits multiple key
gene expression events and effector functions in CD8+
T cells. European Journal of Immunology, 38(9),
2438–2450. https://doi.org/10.1002/eji.200838289
Chang, C. H., Qiu, J., O’Sullivan, D., Buck, M. D.,
Noguchi, T., Curtis, J. D., Chen, Q., Gindin, M., Gubin,
M. M., Van Der Windt, G. J. W., Tonc, E., Schreiber,
R. D., Pearce, E. J., & Pearce, E. L. (2015). Metabolic
Competition in the Tumor Microenvironment Is a
Driver of Cancer Progression. Cell, 162(6), 1229–
1241. https://doi.org/10.1016/j.cell.2015.08.016
Chen, N., Fang, W., Zhan, J., Hong, S., Tang, Y., Kang, S.,
Zhang, Y., He, X., Zhou, T., Qin, T., Huang, Y., Yi, X.,
& Zhang, L. (2015). Upregulation of PD-L1 by EGFR
activation mediates the immune escape in EGFR-
driven NSCLC: Implication for optional immune
targeted therapy for NSCLC patients with EGFR
mutation. Journal of Thoracic Oncology, 10(6), 910–
923. https://doi.org/10.1097/JTO.0000000000000500
Coelho, M. A., de Carné Trécesson, S., Rana, S., Zecchin,
D., Moore, C., Molina-Arcas, M., East, P., Spencer-
Dene, B., Nye, E., Barnouin, K., Snijders, A. P., Lai,
W. S., Blackshear, P. J., & Downward, J. (2017).
Oncogenic RAS Signaling Promotes Tumor
Immunoresistance by Stabilizing PD-L1 mRNA.
Immunity, 47(6), 1083-1099.e6.
https://doi.org/10.1016/j.immuni.2017.11.016
ICBB 2022 - International Conference on Biotechnology and Biomedicine
362

Corbet, C., & Feron, O. (2017). Tumour acidosis: From the
passenger to the driver’s seat. Nature Reviews Cancer,
17(10), 577–593. https://doi.org/10.1038/nrc.2017.77
De Vries, T. J., Fourkour, A., Wobbes, T., Verkroost, G.,
Ruiter, D. J., & Van Muijen, G. N. P. (1997).
Heterogeneous expression of immunotherapy
candidate proteins gp100, MART-1, and tyrosinase in
human melanoma cell lines and in human melanocytic
lesions. Cancer Research, 57(15), 3223–3229.
Deaglio, S., Dwyer, K. M., Gao, W., Friedman, D., Usheva,
A., Erat, A., Chen, J. F., Enjyoji, K., Linden, J., Oukka,
M., Kuchroo, V. K., Strom, T. B., & Robson, S. C.
(2007). Adenosine generation catalyzed by CD39 and
CD73 expressed on regulatory T cells mediates
immune suppression. Journal of Experimental
Medicine, 204(6), 1257–1265.
https://doi.org/10.1084/jem.20062512
Di Giacomo, A. M., Covre, A., Finotello, F., Rieder, D.,
Danielli, R., Sigalotti, L., Giannarelli, D., Petitprez, F.,
Lacroix, L., Valente, M., Cutaia, O., Fazio, C., Amato,
G., Lazzeri, A., Monterisi, S., Miracco, C., Coral, S.,
Anichini, A., Bock, C., … Maio, M. (2019).
Guadecitabine plus ipilimumab in unresectable
melanoma: The NIBIT-M4 clinical trial. Clinical
Cancer Research, 25(24), 7351–7362.
https://doi.org/10.1158/1078-0432.CCR-19-1335
Ding, Q. Q., Chauvin, J. M., & Zarour, H. M. (2020).
Targeting novel inhibitory receptors in cancer
immunotherapy. Seminars in Immunology,
49(December), 101436.
https://doi.org/10.1016/j.smim.2020.101436
Dirix, L. Y., Takacs, I., Jerusalem, G., Nikolinakos, P.,
Arkenau, H. T., Forero-Torres, A., Boccia, R.,
Lippman, M. E., Somer, R., Smakal, M., Emens, L. A.,
Hrinczenko, B., Edenfield, W., Gurtler, J., von
Heydebreck, A., Grote, H. J., Chin, K., & Hamilton, E.
P. (2018). Avelumab, an anti-PD-L1 antibody, in
patients with locally advanced or metastatic breast
cancer: A phase 1b JAVELIN solid tumor study. Breast
Cancer Research and Treatment, 167(3), 671–686.
https://doi.org/10.1007/s10549-017-4537-5
Dunn, G. P., Koebel, C. M., & Schreiber, R. D. (2006).
Interferons, immunity and cancer immunoediting. In
Nature Reviews Immunology (Vol. 6, Issue 11, pp.
836–848). Nat Rev Immunol.
https://doi.org/10.1038/nri1961
Fares, C. M., Van Allen, E. M., Drake, C. G., Allison, J. P.,
& Hu-Lieskovan, S. (2019). Mechanisms of Resistance
to Immune Checkpoint Blockade: Why Does
Checkpoint Inhibitor Immunotherapy Not Work for All
Patients? American Society of Clinical Oncology
Educational Book, 39, 147–164.
https://doi.org/10.1200/edbk_240837
Fridman, W. H., Pagès, F., Saut
̀
s-Fridman, C., & Galon, J.
(2012). The immune contexture in human tumours:
Impact on clinical outcome. Nature Reviews Cancer,
12(4), 298–306. https://doi.org/10.1038/nrc3245
Gallagher, S. J., Shklovskaya, E., & Hersey, P. (2017).
Epigenetic modulation in cancer immunotherapy.
Current Opinion in Pharmacology, 35, 48–56.
https://doi.org/10.1016/j.coph.2017.05.006
Galon, J., & Bruni, D. (2019). Approaches to treat immune
hot, altered and cold tumours with combination
immunotherapies. Nature Reviews Drug Discovery,
18(3), 197–218. https://doi.org/10.1038/s41573-018-
0007-y
Gao, J., Shi, L. Z., Zhao, H., Chen, J., Xiong, L., He, Q.,
Chen, T., Roszik, J., Bernatchez, C., Woodman, S. E.,
Chen, P. L., Hwu, P., Allison, J. P., Futreal, A., Wargo,
J. A., & Sharma, P. (2016). Loss of IFN-γ Pathway
Genes in Tumor Cells as a Mechanism of Resistance to
Anti-CTLA-4 Therapy. Cell, 167(2), 397-404.e9.
https://doi.org/10.1016/j.cell.2016.08.069
Garcia-Diaz, A., Shin, D. S., Moreno, B. H., Saco, J.,
Escuin-Ordinas, H., Rodriguez, G. A., Zaretsky, J. M.,
Sun, L., Hugo, W., Wang, X., Parisi, G., Saus, C. P.,
Torrejon, D. Y., Graeber, T. G., Comin-Anduix, B.,
Hu-Lieskovan, S., Damoiseaux, R., Lo, R. S., & Ribas,
A. (2017). Interferon Receptor Signaling Pathways
Regulating PD-L1 and PD-L2 Expression. Cell
Reports, 19(6), 1189–1201.
https://doi.org/10.1016/j.celrep.2017.04.031
GMÜNDER, H., ECK, H. ‐P, & DRÖGE, W. (1991). Low
membrane transport activity for cystine in resting and
mitogenically stimulated human lymphocyte
preparations and human T cell clones. European
Journal of Biochemistry, 201(1), 113–117.
https://doi.org/10.1111/j.1432-1033.1991.tb16263.x
Gong, J., Chehrazi-Raffle, A., Reddi, S., & Salgia, R.
(2018). Development of PD-1 and PD-L1 inhibitors as
a form of cancer immunotherapy: A comprehensive
review of registration trials and future considerations.
In Journal for ImmunoTherapy of Cancer (Vol. 6, Issue
1). BioMed Central Ltd.
https://doi.org/10.1186/s40425-018-0316-z
Goodman, A. M., Kato, S., Bazhenova, L., Patel, S. P.,
Frampton, G. M., Miller, V., Stephens, P. J., Daniels,
G. A., & Kurzrock, R. (2017a). Tumor mutational
burden as an independent predictor of response to
immunotherapy in diverse cancers. Molecular Cancer
Therapeutics, 16(11), 2598–2608.
https://doi.org/10.1158/1535-7163.MCT-17-0386
Goodman, A. M., Kato, S., Bazhenova, L., Patel, S. P.,
Frampton, G. M., Miller, V., Stephens, P. J., Daniels,
G. A., & Kurzrock, R. (2017b). Tumor mutational
burden as an independent predictor of response to
immunotherapy in diverse cancers. Molecular Cancer
Therapeutics, 16(11), 2598–2608.
https://doi.org/10.1158/1535-7163.MCT-17-0386
Gopalakrishnan, V., Spencer, C. N., Nezi, L., Reuben, A.,
Andrews, M. C., Karpinets, T. V., Prieto, P. A.,
Vicente, D., Hoffman, K., Wei, S. C., Cogdill, A. P.,
Zhao, L., Hudgens, C. W., Hutchinson, D. S., Manzo,
T., Petaccia De Macedo, M., Cotechini, T., Kumar, T.,
Chen, W. S., … Wargo, J. A. (2018). Gut microbiome
modulates response to anti-PD-1 immunotherapy in
melanoma patients. Science, 359(6371), 97–103.
https://doi.org/10.1126/science.aan4236
Mechanisms of Resistance to Cancer Immunotherapy
363

Gromeier, M., Brown, M. C., Zhang, G., Lin, X., Chen, Y.,
Wei, Z., Beaubier, N., Yan, H., He, Y., Desjardins, A.,
Herndon, J. E., Varn, F. S., Verhaak, R. G., Zhao, J.,
Bolognesi, D. P., Friedman, A. H., Friedman, H. S.,
McSherry, F., Muscat, A. M., … Ashley, D. M. (2021).
Very low mutation burden is a feature of inflamed
recurrent glioblastomas responsive to cancer
immunotherapy. Nature Communications, 12(1).
https://doi.org/10.1038/s41467-020-20469-6
Guo, Y., Pan, W., Liu, S., Shen, Z., Xu, Y., & Hu, L.
(2020). ERK/MAPK signalling pathway and
tumorigenesis. Experimental and Therapeutic
Medicine, 19(3), 1997.
https://doi.org/10.3892/etm.2020.8454
Haist, M., Stege, H., Grabbe, S., & Bros, M. (2021).
Review the functional crosstalk between myeloid-
derived suppressor cells and regulatory t cells within
the immunosuppressive tumor microenvironment. In
Cancers (Vol. 13, Issue 2, pp. 1–34). MDPI AG.
https://doi.org/10.3390/cancers13020210
Hansen, A. R., Massard, C., Ott, P. A., Haas, N. B., Lopez,
J. S., Ejadi, S., Wallmark, J. M., Keam, B., Delord, J.
P., Aggarwal, R., Gould, M., Yang, P., Keefe, S. M., &
Piha-Paul, S. A. (2018). Pembrolizumab for advanced
prostate adenocarcinoma: Findings of the KEYNOTE-
028 study. Annals of Oncology, 29(8), 1807–1813.
https://doi.org/10.1093/annonc/mdy232
Hasanali, Z. S., Saroya, B. S., Stuart, A., Shimko, S.,
Evans, J., Shah, M. V., Sharma, K., Leshchenko, V. V.,
Parekh, S., Loughran, T. P., & Epner, E. M. (2015).
Epigenetic therapy overcomes treatment resistance in T
cell prolymphocytic leukemia. Science Translational
Medicine, 7(293).
https://doi.org/10.1126/scitranslmed.aaa5079
Hegde, P. S., & Chen, D. S. (2020). Top 10 Challenges in
Cancer Immunotherapy. In Immunity (Vol. 52, Issue 1,
pp. 17–35). Cell Press.
https://doi.org/10.1016/j.immuni.2019.12.011
Hegde, P. S., Karanikas, V., & Evers, S. (2016). The where,
the when, and the how of immune monitoring for
cancer immunotherapies in the era of checkpoint
inhibition. Clinical Cancer Research, 22(8), 1865–
1874. https://doi.org/10.1158/1078-0432.CCR-15-
1507
Hellmann, M. D., Nathanson, T., Rizvi, H., Creelan, B. C.,
Sanchez-Vega, F., Ahuja, A., Ni, A., Novik, J. B.,
Mangarin, L. M. B., Abu-Akeel, M., Liu, C., Sauter, J.
L., Rekhtman, N., Chang, E., Callahan, M. K., Chaft, J.
E., Voss, M. H., Tenet, M., Li, X. M., … Wolchok, J.
D. (2018). Genomic Features of Response to
Combination Immunotherapy in Patients with
Advanced Non-Small-Cell Lung Cancer. Cancer Cell,
33(5), 843-852.e4.
https://doi.org/10.1016/j.ccell.2018.03.018
Hong, H., Wang, Q., Li, J., Liu, H., Meng, X., & Zhang, H.
(2019). Aging, cancer and immunity. Journal of
Cancer, 10(13), 3021–3027.
https://doi.org/10.7150/jca.30723
Hopkins-Donaldson, S., Ziegler, A., Kurtz, S., Bigosch, C.,
Kandioler, D., Ludwig, C., Zangemeister-Wittke, U., &
Stahel, R. (2003). Silencing of death receptor and
caspase-8 expression in small cell lung carcinoma cell
lines tumors by DNA methylation. Cell Death and
Differentiation, 10(3), 356–364.
https://doi.org/10.1038/sj.cdd.4401157
Hou, A., Hou, K., Huang, Q., Lei, Y., & Chen, W. (2020).
Targeting Myeloid-Derived Suppressor Cell, a
Promising Strategy to Overcome Resistance to Immune
Checkpoint Inhibitors. Frontiers in Immunology,
11(May), 1–19.
https://doi.org/10.3389/fimmu.2020.00783
Hoxhaj, G., & Manning, B. D. (2020). The PI3K–AKT
network at the interface of oncogenic signalling and
cancer metabolism. In Nature Reviews Cancer (Vol.
20, Issue 2, pp. 74–88). Nature Research.
https://doi.org/10.1038/s41568-019-0216-7
Huang, B., Pan, P. Y., Li, Q., Sato, A. I., Levy, D. E.,
Bromberg, J., Divino, C. M., & Chen, S. H. (2006). Gr-
1+CD115+ immature myeloid suppressor cells mediate
the development of tumor-induced T regulatory cells
and T-cell anergy in tumor-bearing host. Cancer
Research, 66(2), 1123–1131.
https://doi.org/10.1158/0008-5472.CAN-05-1299
Hugo, W., Zaretsky, J. M., Sun, L., Song, C., Homet, B.,
Hu-lieskovan, S., Berent-maoz, B., Pang, J.,
Chmielowski, B., Cherry, G., Seja, E., Lomeli, S.,
Kong, X., Kelley, M. C., Sosman, A., Johnson, D. B.,
Ribas, A., & Lo, R. S. (2017). Genomic and
transriptomic features of anti-PD1 response. Cell,
165(1), 35–44.
https://doi.org/10.1016/j.cell.2016.02.065.Genomic
Husain, Z., Huang, Y., Seth, P., & Sukhatme, V. P. (2013).
Tumor-Derived Lactate Modifies Antitumor Immune
Response: Effect on Myeloid-Derived Suppressor Cells
and NK Cells. The Journal of Immunology, 191(3),
1486–1495.
https://doi.org/10.4049/jimmunol.1202702
Insinga, A., Monestiroli, S., Ronzoni, S., Gelmetti, V.,
Marchesi, F., Viale, A., Altucci, L., Nervi, C., Minucci,
S., & Pelicci, P. G. (2005). Inhibitors of histone
deacetylases induce tumor-selective apoptosis through
activation of the death receptor pathway. Nature
Medicine, 11(1), 71–76.
https://doi.org/10.1038/nm1160
Italiani, P., & Boraschi, D. (2014). From monocytes to
M1/M2 macrophages: Phenotypical vs. functional
differentiation. Frontiers in Immunology, 5(OCT), 1–
22. https://doi.org/10.3389/fimmu.2014.00514
Jarnicki, A. G., Lysaght, J., Todryk, S., & Mills, K. H. G.
(2006). Suppression of Antitumor Immunity by IL-
10 and TGF-β-Producing T Cells Infiltrating the
Growing Tumor: Influence of Tumor Environment on
the Induction of CD4 + and CD8 + Regulatory T Cells
. The Journal of Immunology, 177(2), 896–904.
https://doi.org/10.4049/jimmunol.177.2.896
Jenkins, R. W., Barbie, D. A., & Flaherty, K. T. (2018).
Mechanisms of resistance to immune checkpoint
inhibitors. British Journal of Cancer, 118
(1), 9–16.
https://doi.org/10.1038/bjc.2017.434
ICBB 2022 - International Conference on Biotechnology and Biomedicine
364

Kalbasi, A., & Ribas, A. (2020). Tumour-intrinsic
resistance to immune checkpoint blockade. Nature
Reviews Immunology, 20(1), 25–39.
https://doi.org/10.1038/s41577-019-0218-4
Kloepper, J., Riedemann, L., Amoozgar, Z., Seano, G.,
Susek, K., Yu, V., Dalvie, N., Amelung, R. L., Datta,
M., Song, J. W., Askoxylakis, V., Taylor, J. W., Lu-
Emerson, C., Batista, A., Kirkpatrick, N. D., Jung, K.,
Snuderl, M., Muzikansky, A., Stubenrauch, K. G., …
Jain, R. K. (2016). Ang-2/VEGF bispecific antibody
reprograms macrophages and resident microglia to
anti-tumor phenotype and prolongs glioblastoma
survival. Proceedings of the National Academy of
Sciences of the United States of America, 113(16),
4476–4481. https://doi.org/10.1073/pnas.1525360113
Kumagai, S., Togashi, Y., Sakai, C., Kawazoe, A.,
Kawazu, M., Ueno, T., Sato, E., Kuwata, T., Kinoshita,
T., Yamamoto, M., Nomura, S., Tsukamoto, T., Mano,
H., Shitara, K., & Nishikawa, H. (2020). An Oncogenic
Alteration Creates a Microenvironment that Promotes
Tumor Progression by Conferring a Metabolic
Advantage to Regulatory T Cells. Immunity, 53(1),
187-203.e8.
https://doi.org/10.1016/j.immuni.2020.06.016
Kurachi, M. (2019). CD8 + T cell exhaustion. Seminars in
Immunopathology, 41(3), 327–337.
https://doi.org/10.1007/s00281-019-00744-5
Lastwika, K. J., Wilson, W., Li, Q. K., Norris, J., Xu, H.,
Ghazarian, S. R., Kitagawa, H., Kawabata, S., Taube,
J. M., Yao, S., Liu, L. N., Gills, J. J., & Dennis, P. A.
(2016). Control of PD-L1 expression by oncogenic
activation of the AKT-mTOR pathway in non-small
cell lung cancer. Cancer Research, 76(2), 227–238.
https://doi.org/10.1158/0008-5472.CAN-14-3362
Law, A. M. K., Valdes-Mora, F., & Gallego-Ortega, D.
(2020). Myeloid-Derived Suppressor Cells as a
Therapeutic Target for Cancer. Cells, 9(3).
https://doi.org/10.3390/cells9030561
Li, H., Han, Y., Guo, Q., Zhang, M., & Cao, X. (2009).
Cancer-Expanded Myeloid-Derived Suppressor Cells
Induce Anergy of NK Cells through Membrane-Bound
TGF-β1. The Journal of Immunology, 182(1), 240–249.
https://doi.org/10.4049/jimmunol.182.1.240
Liao, W., Overman, M. J., Boutin, A. T., Shang, X., Zhao,
D., Dey, P., Li, J., Wang, G., Lan, Z., Li, J., Tang, M.,
Jiang, S., Ma, X., Chen, P., Katkhuda, R., Korphaisarn,
K., Chakravarti, D., Chang, A., Spring, D. J., …
DePinho, R. A. (2019). KRAS-IRF2 Axis Drives
Immune Suppression and Immune Therapy Resistance
in Colorectal Cancer. Cancer Cell, 35(4), 559-572.e7.
https://doi.org/10.1016/j.ccell.2019.02.008
Limagne, E., Richard, C., Thibaudin, M., Fumet, J. D.,
Truntzer, C., Lagrange, A., Favier, L., Coudert, B., &
Ghiringhelli, F. (2019). Tim-3/galectin-9 pathway and
mMDSC control primary and secondary resistances to
PD-1 blockade in lung cancer patients.
OncoImmunology, 8(4), 1–13.
https://doi.org/10.1080/2162402X.2018.1564505
Lind, H., Gameiro, S. R., Jochems, C., Donahue, R. N.,
Strauss, J., Gulley, J. L., Palena, C., & Schlom, J.
(2020). Dual targeting of TGF-β and PD-L1 via a
bifunctional anti-PD-L1/TGF-βRII agent: Status of
preclinical and clinical advances. In Journal for
ImmunoTherapy of Cancer (Vol. 8, Issue 1). BMJ
Publishing Group. https://doi.org/10.1136/jitc-2019-
000433
Loi, S., Dushyanthen, S., Beavis, P. A., Salgado, R.,
Denkert, C., Savas, P., Combs, S., Rimm, D. L.,
Giltnane, J. M., Estrada, M. V., Sánchez, V., Sanders,
M. E., Cook, R. S., Pilkinton, M. A., Mallal, S. A.,
Wang, K., Miller, V. A., Stephens, P. J., Yelensky, R.,
… Balko, J. M. (2016). RAS/MAPK activation is
associated with reduced tumor-infiltrating lymphocytes
in triple-negative breast cancer: Therapeutic
cooperation between MEK and PD-1/PD-L1 immune
checkpoint inhibitors. Clinical Cancer Research,
22(6), 1499–1509. https://doi.org/10.1158/1078-
0432.CCR-15-1125
Long, L., Zhang, X., Chen, F., Pan, Q., Phiphatwatchara,
P., Zeng, Y., & Chen, H. (2018). The promising
immune checkpoint LAG-3: From tumor
microenvironment to cancer immunotherapy. Genes
and Cancer, 9(5–6), 176–189.
https://doi.org/10.18632/genesandcancer.180
Lukas, R. V., Rodon, J., Becker, K., Wong, E. T., Shih, K.,
Touat, M., Fassò, M., Osborne, S., Molinero, L.,
O’Hear, C., Grossman, W., & Baehring, J. (2018).
Clinical activity and safety of atezolizumab in patients
with recurrent glioblastoma. Journal of Neuro-
Oncology, 140(2), 317–328.
https://doi.org/10.1007/s11060-018-2955-9
Luke, J. J., Bao, R., Sweis, R. F., Spranger, S., & Gajewski,
T. F. (2019). WNT/b-catenin pathway activation
correlates with immune exclusion across human
cancers. Clinical Cancer Research, 25(10), 3074–
3083. https://doi.org/10.1158/1078-0432.CCR-18-
1942
Luo, N., Nixon, M. J., Gonzalez-Ericsson, P. I., Sanchez,
V., Opalenik, S. R., Li, H., Zahnow, C. A., Nickels, M.
L., Liu, F., Tantawy, M. N., Sanders, M. E., Manning,
H. C., & Balko, J. M. (2018). DNA methyltransferase
inhibition upregulates MHC-I to potentiate cytotoxic T
lymphocyte responses in breast cancer. Nature
Communications, 9(1), 1–11.
https://doi.org/10.1038/s41467-017-02630-w
Luu, M., Riester, Z., Baldrich, A., Reichardt, N., Yuille, S.,
Busetti, A., Klein, M., Wempe, A., Leister, H., Raifer,
H., Picard, F., Muhammad, K., Ohl, K., Romero, R.,
Fischer, F., Bauer, C. A., Huber, M., Gress, T. M.,
Lauth, M., … Steinhoff, U. (2021). Microbial short-
chain fatty acids modulate CD8+ T cell responses and
improve adoptive immunotherapy for cancer. Nature
Communications, 1–12.
https://doi.org/10.1038/s41467-021-24331-1
Madsen, R. R., Vanhaesebroeck, B., & Semple, R. K.
(2018). Cancer-Associated PIK3CA Mutations in
Overgrowth Disorders. Trends in Molecular Medicine,
24(10), 856–870.
https://doi.org/10.1016/j.molmed.2018.08.003
Mechanisms of Resistance to Cancer Immunotherapy
365

Maio, M., Covre, A., Fratta, E., Di Giacomo, A. M.,
Taverna, P., Natali, P. G., Coral, S., & Sigalotti, L.
(2015). Molecular pathways: At the crossroads of
cancer epigenetics and immunotherapy. Clinical
Cancer Research, 21(18), 4040–4047.
https://doi.org/10.1158/1078-0432.CCR-14-2914
Mariathasan, S., Turley, S. J., Nickles, D., Castiglioni, A.,
Yuen, K., Wang, Y., Kadel, E. E., Koeppen, H.,
Astarita, J. L., Cubas, R., Jhunjhunwala, S.,
Banchereau, R., Yang, Y., Guan, Y., Chalouni, C., Ziai,
J., Şenbabaoǧlu, Y., Santoro, S., Sheinson, D., …
Powles, T. (2018). TGFβ attenuates tumour response to
PD-L1 blockade by contributing to exclusion of T cells.
Nature, 554(7693), 544–548.
https://doi.org/10.1038/nature25501
Matson, V., Fessler, J., Bao, R., Chongsuwat, T., Zha, Y.,
Alegre, M. L., Luke, J. J., & Gajewski, T. F. (2018).
The commensal microbiome is associated with anti-
PD-1 efficacy in metastatic melanoma patients.
Science, 359(6371), 104–108.
https://doi.org/10.1126/science.aao3290
Maude, S. L., Frey, N., Shaw, P. A., Aplenc, R., Barrett, D.
M., Bunin, N. J., Chew, A., Gonzalez, V. E., Zheng, Z.,
Lacey, S. F., Mahnke, Y. D., Melenhorst, J. J.,
Rheingold, S. R., Shen, A., Teachey, D. T., Levine, B.
L., June, C. H., Porter, D. L., & Grupp, S. A. (2014).
Chimeric Antigen Receptor T Cells for Sustained
Remissions in Leukemia. New England Journal of
Medicine, 371(16), 1507–1517.
https://doi.org/10.1056/nejmoa1407222
Mcgranahan, N., Furness, A. J. S., Rosenthal, R., Lyngaa,
R., Saini, S. K., Jamal-hanjani, M., Gareth, A., Birkbak,
N. J., Hiley, C. T., Watkins, T. B. K., Shafi, S.,
Murugaesu, N., Mitter, R., Akarca, A. U., Linares, J.,
Henry, J. Y., Allen, E. M. Van, Miao, D., & Schilling,
B. (2016). Clonal neoantigens elicit T cell
immunoreactivity and sensitivity to immune
checkpoint blockade. Science, 351(6280), 1463–1469.
https://doi.org/10.1126/science.aaf1490.Clonal
Mcgranahan, N., Rosenthal, R., Hiley, C. T., Herrero, J., &
Swanton, C. (2017). Allele-Specific HLA Loss and
Immune Escape in Lung Cancer Evolution. Cell,
171(6), 1259–1271.
https://doi.org/https://doi.org/10.1016/j.cell.2017.10.0
01
Meissner, M., Reichert, T. E., Kunkel, M., Gooding, W.,
Whiteside, T. L., Ferrone, S., & Seliger, B. (2005).
Defects in the human leukocyte antigen class I antigen-
processing machinery in head and neck squamous cell
carcinoma: Association with clinical outcome. Clinical
Cancer Research, 11(7), 2552–2560.
https://doi.org/10.1158/1078-0432.CCR-04-2146
Mo, X., Zhang, H., Preston, S., Martin, K., Zhou, B.,
Vadalia, N., Gamero, A. M., Soboloff, J., Tempera, I.,
& Zaidi, M. R. (2018). Interferon-γ Signaling in
Melanocytes and Melanoma Cells Regulates
Expression of CTLA-4. Cancer Research, 78(2), 436–
450. https://doi.org/10.1158/0008-5472.CAN-17-1615
Mosser, D. M., & Edwards, J. P. (2008). Exploring the full
spectrum of macrophage activation. Nature Reviews
Immunology, 8(12), 958–969.
https://doi.org/10.1038/nri2448
Müller, B., Fischer, B., & Kreutz, W. (2000). An acidic
microenvironment impairs the generation of non-major
histocompatibility complex-restricted killer cells.
Immunology, 99(3), 375–384.
https://doi.org/10.1046/j.1365-2567.2000.00975.x
Munder, M., Schneider, H., Luckner, C., Giese, T.,
Langhans, C. D., Fuentes, J. M., Kropf, P., Mueller, I.,
Kolb, A., Modolell, M., & Ho, A. D. (2006).
Suppression of T-cell functions by human granulocyte
arginase. Blood, 108(5), 1627–1634.
https://doi.org/10.1182/blood-2006-11-010389
Nagarsheth, N., Peng, D., Kryczek, I., Wu, K., Li, W.,
Zhao, E., Zhao, L., Wei, S., Frankel, T., Vatan, L.,
Szeliga, W., Dou, Y., Owens, S., Marquez, V., Tao, K.,
Huang, E., Wang, G., & Zou, W. (2016). PRC2
epigenetically silences Th1-type chemokines to
suppress effector T-cell trafficking in colon cancer.
Cancer Research, 76(2), 275–282.
https://doi.org/10.1158/0008-5472.CAN-15-1938
Najjar, Y. G., Rayman, P., Jia, X., Pavicic, P. G., Rini, B.
I., Tannenbaum, C., Ko, J., Haywood, S., Cohen, P.,
Hamilton, T., Diaz-Montero, C. M., & Finke, J. (2017).
Myeloid-derived suppressor cell subset accumulation
in renal cell carcinoma parenchyma is associated with
intratumoral expression of IL1b, IL8, CXCL5, and
Mip-1α. Clinical Cancer Research, 23(9), 2346–2355.
https://doi.org/10.1158/1078-0432.CCR-15-1823
Ni, L., & Lu, J. (2018). Interferon gamma in cancer
immunotherapy. In Cancer Medicine (Vol. 7, Issue 9,
pp. 4509–4516). Blackwell Publishing Ltd.
https://doi.org/10.1002/cam4.1700
Nowicki, T. S., Hu-Lieskovan, S., & Ribas, A. (2018).
Mechanisms of Resistance to PD-1 and PD-L1
Blockade. In Cancer Journal (United States) (Vol. 24,
Issue 1, pp. 47–53). Lippincott Williams and Wilkins.
https://doi.org/10.1097/PPO.0000000000000303
Pauken, K. E., Sammons, M. A., Odorizzi, P. M., Manne,
S., Godec, J., Khan, O., Drake, A. M., Chen, Z., Sen,
D. R., Kurachi, M., Barnitz, R. A., Bartman, C.,
Bengsch, B., Huang, A. C., Schenkel, J. M., Vahedi,
G., Haining, W. N., Berger, S. L., & Wherry, E. J.
(2016). Epigenetic stability of exhausted T cells limits
durability of reinvigoration by PD-1 blockade. Science,
354(6316), 1160–1165.
https://doi.org/10.1126/science.aaf2807
Peng, D., Kryczek, I., Nagarsheth, N., Zhao, L., Wei, S.,
Wang, W., Sun, Y., Zhao, E., Vatan, L., Szeliga, W.,
Kotarski, J., Tarkowski, R., Dou, Y., Cho, K., Hensley-
Alford, S., Munkarah, A., Liu, R., & Zou, W. (2015).
Epigenetic silencing of TH1-type chemokines shapes
tumour immunity and immunotherapy. Nature,
527(7577), 249–253.
https://doi.org/10.1038/nature15520
Peng, W., Chen, J. Q., Liu, C., Malu, S., Creasy, C.,
Tetzlaff, M. T., Xu, C., McKenzie, J. A., Zhang, C.,
Liang, X., Williams, L. J., Deng, W., Chen, G.,
Mbofung, R., Lazar, A. J., Torres-Cabala, C. A.,
Cooper, Z. A., Chen, P.-L., Tieu, T. N., … Hwu, P.
ICBB 2022 - International Conference on Biotechnology and Biomedicine
366

(2016). Loss of PTEN Promotes Resistance to T Cell–
Mediated Immunotherapy. Cancer Discovery, 6(2),
202–216. https://doi.org/10.1158/2159-8290.CD-15-
0283
Perumal, D., Imai, N., Lagana, A., Finnigan, J., Melnekoff,
D., Leshchenko, V. V., Solovyov, A., Madduri, D.,
Chari, A., Cho, H. J., Dudley, J. T., Brody, J. D.,
Jagannath, S., Greenbaum, B., Gnjatic, S., Bhardwaj,
N., & Parekh, S. (2020). Mutation-derived Neoantigen-
specific T-cell Responses in Multiple Myeloma.
Clinical Cancer Research, 26(2), 450–464.
https://doi.org/10.1158/1078-0432.CCR-19-2309
Porta, C., Paglino, C., & Mosca, A. (2014). Targeting
PI3K/Akt/mTOR signaling in cancer. In Frontiers in
Oncology: Vol. 4 APR. Frontiers Research Foundation.
https://doi.org/10.3389/fonc.2014.00064
Rech, A. J., Mick, R., Martin, S., Recio, A., Aqui, N. A.,
Powell, D. J., Colligon, T. A., Trosko, J. A., Leinbach,
L. I., Pletcher, C. H., Tweed, C. K., DeMichele, A.,
Fox, K. R., Domchek, S. M., Riley, J. L., &
Vonderheide, R. H. (2012). CD25 blockade depletes
and selectively reprograms regulatory T cells in concert
with immunotherapy in cancer patients. Science
Translational Medicine, 4(134), 134ra62.
https://doi.org/10.1126/scitranslmed.3003330
Reeves, E., & James, E. (2017). Antigen processing and
immune regulation in the response to tumours.
Immunology, 150(1), 16–24.
https://doi.org/10.1111/imm.12675
Reinfeld, B. I., Madden, M. Z., Wolf, M. M., Chytil, A.,
Bader, J. E., Patterson, A. R., Sugiura, A., Cohen, A.
S., Ali, A., Do, B. T., Muir, A., Lewis, C. A., Hongo,
R. A., Young, K. L., Brown, R. E., Todd, V. M.,
Huffstater, T., Abraham, A., O’Neil, R. T., …
Rathmell, W. K. (2021). Cell-programmed nutrient
partitioning in the tumour microenvironment. Nature,
593(7858), 282–288. https://doi.org/10.1038/s41586-
021-03442-1
Reisländer, T., Lombardi, E. P., Groelly, F. J., Miar, A.,
Porru, M., Di Vito, S., Wright, B., Lockstone, H.,
Biroccio, A., Harris, A., Londoño-Vallejo, A., &
Tarsounas, M. (2019). BRCA2 abrogation triggers
innate immune responses potentiated by treatment with
PARP inhibitors. Nature Communications, 10(1),
3143. https://doi.org/10.1038/s41467-019-11048-5
Ribas, A., & Wolchok, J. D. (2018). Cancer
immunotherapy using checkpoint blockade. In Science
(Vol. 359, Issue 6382, pp. 1350–1355). American
Association for the Advancement of Science.
https://doi.org/10.1126/science.aar4060
Rizvi, N. A., Hellmann, M. D., Snyder, A., Kvistborg, P.,
Makarov, V., Havel, J. J., Lee, W., Yuan, J., Wong, P.,
Ho, T. S., Miller, M. L., Rekhtman, N., Moreira, A. L.,
Ibrahim, F., Bruggeman, C., Gasmi, B., Zappasodi, R.,
Maeda, Y., Sander, C., … Chan, T. A. (2015).
Mutational landscape determines sensitivity to PD-1
blockade in non-small cell lung cancer. Science,
348(6230), 124–128.
https://doi.org/10.1126/science.aaa1348
Rodriguez, P. C., Quiceno, D. G., & Ochoa, A. C. (2007).
L-arginine availability regulates T-lymphocyte cell-
cycle progression. Blood, 109(4), 1568–1573.
https://doi.org/10.1182/blood-2006-06-031856
Rosenthal, R., Cadieux, E. L., Salgado, R., Bakir, M. Al,
Moore, D. A., Hiley, C. T., Lund, T., Tanić, M.,
Reading, J. L., Joshi, K., Henry, J. Y., Ghorani, E.,
Wilson, G. A., Nicolai, J., Hellmann, M. D., Feber, A.,
Chain, B., & Herrero, J. (2020). Neoantigen directed
immune escape in lung cancer evolution. Nature,
567(7749), 479–485. https://doi.org/10.1038/s41586-
019-1032-7.Neoantigen
Rotte, A. (2019). Combination of CTLA-4 and PD-1
blockers for treatment of cancer. In Journal of
Experimental and Clinical Cancer Research (Vol. 38,
Issue 1). BioMed Central Ltd.
https://doi.org/10.1186/s13046-019-1259-z
Ryzhov, S., Novitskiy, S. V., Goldstein, A. E., Biktasova,
A., Blackburn, M. R., Biaggioni, I., Dikov, M. M., &
Feoktistov, I. (2011). Adenosinergic Regulation of
the Expansion and Immunosuppressive Activity of
CD11b + Gr1 + Cells . The Journal of Immunology,
187(11), 6120–6129.
https://doi.org/10.4049/jimmunol.1101225
Sahin, U., Derhovanessian, E., Miller, M., Kloke, B.-P.,
Simon, P., Löwer, M., Bukur, V., Tadmor, A. D.,
Luxemburger, U., Schrörs, B., Omokoko, T., Vormehr,
M., Albrecht, C., Paruzynski, A., Kuhn, A. N., Buck,
J., Heesch, S., Schreeb, K. H., Müller, F., … Türeci, Ö.
(2017). Personalized RNA mutanome vaccines
mobilize poly-specific therapeutic immunity against
cancer. Nature, 547(7662), 222–226.
https://doi.org/10.1038/nature23003
Sakuishi, K., Apetoh, L., Sullivan, J. M., Blazar, B. R.,
Kuchroo, V. K., & Anderson, A. C. (2010). Targeting
Tim-3 and PD-1 pathways to reverse T cell exhaustion
and restore anti-tumor immunity. Journal of
Experimental Medicine, 207(10), 2187–2194.
https://doi.org/10.1084/jem.20100643
Sasidharan Nair, V., El Salhat, H., Taha, R. Z., John, A.,
Ali, B. R., & Elkord, E. (2018). DNA methylation and
repressive H3K9 and H3K27 trimethylation in the
promoter regions of PD-1, CTLA-4, TIM-3, LAG-3,
TIGIT, and PD-L1 genes in human primary breast
cancer. Clinical Epigenetics, 10(1), 1–12.
https://doi.org/10.1186/s13148-018-0512-1
Schumacher, T. N., & Schreiber, R. D. (2015). Neoantigens
in cancer immunotherapy. Science, 348(6230), 69–74.
https://doi.org/10.1126/science.aaa4971
Seidel, J. A., Otsuka, A., & Kabashima, K. (2018). Anti-
PD-1 and anti-CTLA-4 therapies in cancer:
Mechanisms of action, efficacy, and limitations.
Frontiers in Oncology, 8(MAR), 1–14.
https://doi.org/10.3389/fonc.2018.00086
Sek, K., Mølck, C., Stewart, G. D., Kats, L., Darcy, P. K.,
& Beavis, P. A. (2018). Targeting adenosine receptor
signaling in cancer immunotherapy. International
Journal of Molecular Sciences, 19(12).
https://doi.org/10.3390/ijms19123837
Mechanisms of Resistance to Cancer Immunotherapy
367

SELIGER, B., DUNN, T., SCHWENZER, A., CASPER,
J., HUBER, C., & SCHMOLL, H. J. (1997). Analysis
of the MHC Class I Antigen Presentation Machinery in
Human Embryonal Carcinomas: Evidence for
Deficiencies in TAP, LMP and MHC Class I
Expression and their Upregulation by IFN‐γ.
Scandinavian Journal of Immunology, 46(6), 625–632.
https://doi.org/10.1046/j.1365-3083.1997.d01-176.x
Setiadi, A. F., David, M. D., Seipp, R. P., Hartikainen, J.
A., Gopaul, R., & Jefferies, W. A. (2007). Epigenetic
Control of the Immune Escape Mechanisms in
Malignant Carcinomas. Molecular and Cellular
Biology, 27(22), 7886–7894.
https://doi.org/10.1128/mcb.01547-07
Sharma, P., Pachynski, R. K., Narayan, V., Fléchon, A.,
Gravis, G., Galsky, M. D., Mahammedi, H., Patnaik,
A., Subudhi, S. K., Ciprotti, M., Simsek, B., Saci, A.,
Hu, Y., Han, G. C., & Fizazi, K. (2020). Nivolumab
Plus Ipilimumab for Metastatic Castration-Resistant
Prostate Cancer: Preliminary Analysis of Patients in the
CheckMate 650 Trial. Cancer Cell, 38(4), 489-499.e3.
https://doi.org/10.1016/j.ccell.2020.08.007
Shin, D. S., Zaretsky, J. M., Escuin-Ordinas, H., Garcia-
Diaz, A., Hu-Lieskovan, S., Kalbasi, A., Grasso, C. S.,
Hugo, W., Sandoval, S., Torrejon, D. Y., Palaskas, N.,
Abril-Rodriguez, G., Parisi, G., Azhdam, A.,
Chmielowski, B., Cherry, G., Seja, E., Berent-Maoz,
B., Shintaku, I. P., … Ribas, A. (2017). Primary
resistance to PD-1 blockade mediated by JAK1/2
mutations. Cancer Discovery, 7(2), 188–201.
https://doi.org/10.1158/2159-8290.CD-16-1223
Sivan, A., Corrales, L., Hubert, N., Williams, J. B., Aquino-
Michaels, K., Earley, Z. M., Benyamin, F. W., Lei, Y.
M., Jabri, B., Alegre, M. L., Chang, E. B., & Gajewski,
T. F. (2015). Commensal Bifidobacterium promotes
antitumor immunity and facilitates anti-PD-L1
efficacy. Science, 350(6264), 1084–1089.
https://doi.org/10.1126/science.aac4255
Snahnicanova, Z., Kasubova, I., Kalman, M., Grendar, M.,
Mikolajcik, P., Gabonova, E., Laca, L., Caprnda, M.,
Rodrigo, L., Ciccocioppo, R., Kruzliak, P., Plank, L.,
& Lasabova, Z. (2020). Genetic and epigenetic analysis
of the beta-2-microglobulin gene in microsatellite
instable colorectal cancer. Clinical and Experimental
Medicine, 20(1), 87–95.
https://doi.org/10.1007/s10238-019-00601-7
Snyder, A., Makarov, V., Merghoub, T., Yuan, J., Zaretsky,
J. M., Desrichard, A., Walsh, L. A., Postow, M. A.,
Wong, P., Ho, T. S., Hollmann, T. J., Bruggeman, C.,
Kannan, K., Li, Y., Elipenahli, C., Liu, C., Harbison,
C. T., Wang, L., Ribas, A., … Chan, T. A. (2014).
Genetic Basis for Clinical Response to CTLA-4
Blockade in Melanoma. New England Journal of
Medicine, 371(23), 2189–2199.
https://doi.org/10.1056/nejmoa1406498
Spranger, S., Bao, R., & Gajewski, T. F. (2015).
Melanoma-intrinsic β-catenin signalling prevents anti-
tumour immunity. Nature
, 523(7559), 231–235.
https://doi.org/10.1038/nature14404
Spranger, S., Koblish, H. K., Horton, B., Scherle, P. A.,
Newton, R., & Gajewski, T. F. (2014). Mechanism of
tumor rejection with doublets of CTLA-4, PD-1/PD-
L1, or IDO blockade involves restored IL-2 production
and proliferation of CD8+ T cells directly within the
tumor microenvironment. Journal for ImmunoTherapy
of Cancer, 2(1), 1–14. https://doi.org/10.1186/2051-
1426-2-3
Srivastava, M. K., Sinha, P., Clements, V. K., Rodriguez,
P., & Ostrand-Rosenberg, S. (2010). Myeloid-derived
suppressor cells inhibit T-cell activation by depleting
cystine and cysteine. Cancer Research, 70(1), 68–77.
https://doi.org/10.1158/0008-5472.CAN-09-2587
Stary, G., Olive, A., Radovic-Moreno, A. F., Gondek, D.,
Alvarez, D., Basto, P. A., Perro, M., Vrbanac, V. D.,
Tager, A. M., Shi, J., Yethon, J. A., Farokhzad, O. C.,
Langer, R., Starnbach, M. N., & von Andrian, U. H.
(2015). VACCINES. A mucosal vaccine against
Chlamydia trachomatis generates two waves of
protective memory T cells. Science (New York, N.Y.),
348(6241), aaa8205.
https://doi.org/10.1126/science.aaa8205
Strickler, J. H., Hanks, B. A., & Khasraw, M. (2021).
Tumor mutational burden as a predictor of
immunotherapy response: Is more always better? In
Clinical Cancer Research (Vol. 27, Issue 5, pp. 1236–
1241). American Association for Cancer Research Inc.
https://doi.org/10.1158/1078-0432.CCR-20-3054
Sumimoto, H., Takano, A., Teramoto, K., & Daigo, Y.
(2016). RAS-mitogen-activated protein kinase signal is
required for enhanced PD-L1 expression in human lung
cancers. PLoS ONE, 11(11), 1–18.
https://doi.org/10.1371/journal.pone.0166626
Takeuchi, Y., & Nishikawa, H. (2016). Roles of regulatory
T cells in cancer immunity. International Immunology,
28(8), 401–409.
https://doi.org/10.1093/intimm/dxw025
Turnis, M. E., Sawant, D. V., Szymczak-Workman, A. L.,
Andrews, L. P., Delgoffe, G. M., Yano, H., Beres, A.
J., Vogel, P., Workman, C. J., & Vignali, D. A. A.
(2016). Interleukin-35 Limits Anti-Tumor Immunity.
Immunity, 44(2), 316–329.
https://doi.org/10.1016/j.immuni.2016.01.013
Veldman, J., Visser, L., Berg, A. van den, & Diepstra, A.
(2020). Primary and acquired resistance mechanisms to
immune checkpoint inhibition in Hodgkin lymphoma.
In Cancer Treatment Reviews (Vol. 82). W.B. Saunders
Ltd. https://doi.org/10.1016/j.ctrv.2019.101931
Vétizou, M., Pitt, J. M., Daillère, R., Lepage, P.,
Waldschmitt, N., Flament, C., Rusakiewicz, S., Routy,
B., Roberti, M. P., Duong, C. P. M., Poirier-Colame,
V., Roux, A., Becharef, S., Formenti, S., Golden, E.,
Cording, S., Eberl, G., Schlitzer, A., Ginhoux, F., …
Zitvogel, L. (2015). Anticancer immunotherapy by
CTLA-4 blockade relies on the gut microbiota. Science,
350(6264), 1079–1084.
https://doi.org/10.1126/science.aad1329
Voron, T., Marcheteau, E., Pernot, S., Colussi, O., Tartour,
E., Taieb, J., & Terme, M. (2014). Control of the
immune response by pro-angiogenic factors. Frontiers
ICBB 2022 - International Conference on Biotechnology and Biomedicine
368

in Oncology, 4 APR(April), 1–9.
https://doi.org/10.3389/fonc.2014.00070
Wallis, C. J. D., Butaney, M., Satkunasivam, R., Freedland,
S. J., Patel, S. P., Hamid, O., Pal, S. K., & Klaassen, Z.
(2019). Association of Patient Sex with Efficacy of
Immune Checkpoint Inhibitors and Overall Survival in
Advanced Cancers: A Systematic Review and Meta-
analysis. JAMA Oncology, 5(4), 529–536.
https://doi.org/10.1001/jamaoncol.2018.5904
Wang, J., Li, D., Cang, H., & Guo, B. (2019). Crosstalk
between cancer and immune cells: Role of tumor-
associated macrophages in the tumor
microenvironment. Cancer Medicine, 8(10), 4709–
4721. https://doi.org/10.1002/cam4.2327
Warburg, O. (1956). On the origin of cancer cells. Science,
123(3191), 309–314.
https://doi.org/10.1126/science.123.3191.309
Warburg, O., Wind, F., & Negelein, E. (1927). THE
METABOLISM OF TUMORS IN THE BODY.
Journal of General Physiology, 8(6), 519–530.
https://doi.org/10.1085/jgp.8.6.519
Weber, J. S., Kudchadkar, R. R., Yu, B., Gallenstein, D.,
Horak, C. E., Inzunza, H. D., Zhao, X., Martinez, A. J.,
Wang, W., Gibney, G., Kroeger, J., Eysmans, C.,
Sarnaik, A. A., & Chen, Y. A. (2013). Safety, efficacy,
and biomarkers of nivolumab with vaccine in
ipilimumab-refractory or -naive melanoma. Journal of
Clinical Oncology, 31(34), 4311–4318.
https://doi.org/10.1200/JCO.2013.51.4802
Wylie, B., Chee, J., Forbes, C. A., Booth, M., Stone, S. R.,
Buzzai, A., Abad, A., Foley, B., Cruickshank, M. N., &
Waithman, J. (2019). Acquired resistance during
adoptive cell therapy by transcriptional silencing of
immunogenic antigens. OncoImmunology, 8(8), 1–11.
https://doi.org/10.1080/2162402X.2019.1609874
Xiong, D., Wang, Y., Singavi, A. K., Mackinnon, A. C.,
George, B., & You, M. (2018). Immunogenomic
Landscape Contributes to Hyperprogressive Disease
after Anti-PD-1 Immunotherapy for Cancer. IScience,
9, 258–277. https://doi.org/10.1016/j.isci.2018.10.021
Yaguchi, T., Goto, Y., Kido, K., Mochimaru, H., Sakurai,
T., Tsukamoto, N., Kudo-Saito, C., Fujita, T.,
Sumimoto, H., & Kawakami, Y. (2012). Immune
Suppression and Resistance Mediated by Constitutive
Activation of Wnt/β-Catenin Signaling in Human
Melanoma Cells. The Journal of Immunology, 189(5),
2110–2117.
https://doi.org/10.4049/jimmunol.1102282
Yan, C., Yang, J., Saleh, N., Chen, S.-C., Ayers, G. D.,
Abramson, V. G., Mayer, I. A., & Richmond, A.
(2021). Inhibition of the PI3K/mTOR Pathway in
Breast Cancer to Enhance Response to Immune
Checkpoint Inhibitors in Breast Cancer. International
Journal of Molecular Sciences, 22(10), 5207.
https://doi.org/10.3390/ijms22105207
Yang, F., Markovic, S. N., Molina, J. R., Halfdanarson, T.
R., Pagliaro, L. C., Chintakuntlawar, A. V., Li, R., Wei,
J., Wang, L., Liu, B., Nowakowski, G. S., Wang, M. L.,
& Wang, Y. (2020). Association of Sex, Age, and
Eastern Cooperative Oncology Group Performance
Status with Survival Benefit of Cancer Immunotherapy
in Randomized Clinical Trials: A Systematic Review
and Meta-analysis. JAMA Network Open, 3(8), 1–11.
https://doi.org/10.1001/jamanetworkopen.2020.12534
Yang, H., Zhang, Q., Xu, M., Wang, L., Chen, X., Feng,
Y., Li, Y., Zhang, X., Cui, W., & Jia, X. (2020). CCL2-
CCR2 axis recruits tumor associated macrophages to
induce immune evasion through PD-1 signaling in
esophageal carcinogenesis. Molecular Cancer, 19(1),
41. https://doi.org/10.1186/s12943-020-01165-x
Yang, Z., Zhang, B., Li, D., Lv, M., Huang, C., Shen, G.
X., & Huang, B. (2010). Mast cells mobilize myeloid-
derived suppressor cells and Treg cells in tumor
microenvironment via IL-17 pathway in murine
hepatocarcinoma model. PLoS ONE, 5(1), 1–9.
https://doi.org/10.1371/journal.pone.0008922
Yu, J., Du, W., Yan, F., Wang, Y., Li, H., Cao, S., Yu, W.,
Shen, C., Liu, J., & Ren, X. (2013). Myeloid-Derived
Suppressor Cells Suppress Antitumor Immune
Responses through IDO Expression and Correlate with
Lymph Node Metastasis in Patients with Breast
Cancer. The Journal of Immunology, 190(7), 3783–
3797. https://doi.org/10.4049/jimmunol.1201449
Zakharia, Y., McWilliams, R. R., Rixe, O., Drabick, J.,
Shaheen, M. F., Grossmann, K. F., Kolhe, R.,
Pacholczyk, R., Sadek, R., Tennant, L. L., Smith, C.
M., Kennedy, E. P., Link, C. J., Vahanian, N. N., Yu,
J., Shen, S. S., Brincks, E. L., Rossi, G. R., Munn, D.,
& Milhem, M. (2021). Phase II trial of the IDO
pathway inhibitor indoximod plus pembrolizumab for
the treatment of patients with advanced melanoma.
Journal for ImmunoTherapy of Cancer, 9(6).
https://doi.org/10.1136/jitc-2020-002057
Zaretsky, J. M., Garcia-Diaz, A., Shin, D. S., Escuin-
Ordinas, H., Hugo, W., Hu-Lieskovan, S., Torrejon, D.
Y., Abril-Rodriguez, G., Sandoval, S., Barthly, L.,
Saco, J., Homet Moreno, B., Mezzadra, R.,
Chmielowski, B., Ruchalski, K., Shintaku, I. P.,
Sanchez, P. J., Puig-Saus, C., Cherry, G., … Ribas, A.
(2016). Mutations Associated with Acquired
Resistance to PD-1 Blockade in Melanoma. New
England Journal of Medicine, 375(9), 819–829.
https://doi.org/10.1056/nejmoa1604958
Zhang, Y., & Wang, X. (2020). Targeting the Wnt/β-
catenin signaling pathway in cancer. In Journal of
Hematology and Oncology (Vol. 13, Issue 1). BioMed
Central Ltd. https://doi.org/10.1186/s13045-020-
00990-3
Mechanisms of Resistance to Cancer Immunotherapy
369
