
Therapeutic Cancer Vaccines: Mechanism and Clinical Studies
Xiaojun Chen
University of California, Irvine, U.S.A.
Keywords:
Immunotherapy, Cancer, Vaccines.
Abstract:
Cancer, a genetic disease involved in abnormal cell growth and division, is a major cause of mortality
worldwide for centuries. Among various existing cancer treatments, the development of cancer
immunotherapy has been one of the most popular subjects in the field of oncology. Therapeutic cancer
vaccine, an exciting innovation in cancer immunotherapy, has effectively improved the clinical outcome in
patients by overcoming cancer treating barriers that other cancer treatments such as chemotherapy and
radiation cannot achieve, in which tumor cells that are resistant to traditional cancer treatments continue to
proliferate uncontrollably and invade other tissues causing disease relapse or metastases, thus resulting in
toxicity and collateral destruction to normal tissues and affecting patient’s quality of life. The development
of therapeutic cancer vaccines involves numerous factors and requires prudent choice for each step of the
design. This review provides an overview of basic components of therapeutic cancer vaccines including target
antigens and vaccine platforms and analysis of FDA approved therapeutic cancer vaccines as well as those
currently undergoing clinical trials.
1 INTRODUCTION
Cancer is a disease in which body cells abnormally
grow and proliferate and generally develop into
tumors. It is caused by genetic mutations due to cell
division malfunctions, damages to DNA, or genetic
inheritance. Researchers have identified three major
cancer driver genes including proto-oncogenes
involved in normal cell growth and division (Romei
et al, 2016), tumor suppressor genes involved in
regulating cell division and replication (Wang et al,
2019), and DNA repair genes involved in fixing
damaged DNA (Ronen et al, 2001).
The study of cancer therapy has been one of the
most important and popular subjects in the field of
oncology since the 1930s. However, according to the
World Health Organization, there were
approximately 10 million cancer deaths worldwide in
2020 (WHO, 2021). Thus, the development of more
effective cancer treatments is still in urgent need.
Cancer immunotherapy, also known as immuno-
oncology, is one of various existing cancer treatments
that has significantly developed during the past
decades. It aims to prevent, control, and eradicate
cancer by restoring the activity of the patient's own
immune system in which the immune system is
educated to identify and attack specific cancer cells,
immune cells are enhanced to assist cancer
elimination, and the body is provided with additional
components to boost immune responses. Types of
cancer immunotherapy include cancer vaccines
(therapeutic and prophylactic), adjuvants, tumor-
infecting viruses, targeted antibodies, cytokines,
adoptive cell transfer, and checkpoint inhibitors (CRI,
2020). Among the aforementioned cancer
immunotherapies, cancer vaccines development has
been a rapidly growing field of cancer
immunotherapy research since 1990 DeMaria and
Bilusic, 2019).
Cancer vaccines are classified into two categories:
prophylactic and therapeutic cancer vaccines.
Prophylactic cancer vaccines, or preventive cancer
vaccines, are designed to reduce incidence and
morbidity of cancers caused by oncoviruses and has
mechanism similar to normal infectious disease
vaccines in which an inactivated or weakened form of
the disease is introduced to the immune system in
order to educate the immune system to recognize and
eradicate the disease based on their specific antigens,
thus preventing the patient from the disease
(Vanderbilt, 2020). Since prophylactic cancer
vaccines work best in the preventive setting, patients
must receive the vaccine before viral infection. Two
types of prophylactic cancer vaccines are widely
Chen, X.
Therapeutic Cancer Vaccines: Mechanism and Clinical Studies.
DOI: 10.5220/0012021300003633
In Proceedings of the 4th International Conference on Biotechnology and Biomedicine (ICBB 2022), pages 377-385
ISBN: 978-989-758-637-8
Copyright
c
2023 by SCITEPRESS – Science and Technology Publications, Lda. Under CC license (CC BY-NC-ND 4.0)
377
used: HPV vaccine (Cervarix, Gardasil, and
Gardasil-9) which protects against the human
papillomavirus and prevents cervical, vaginal, vulvar,
and anal cancer in women; HBV vaccine (Heplisav-
B) which protects against the hepatitis B virus and
prevents liver cancer (DeMaria and Bilusic, 2019).
In contrast to prophylactic cancer vaccines,
therapeutic or curative cancer vaccines are
administered to patients with existing malignancy.
Therapeutic cancer vaccine comes in two forms, and
its mechanism involves utilizing the adjuvant
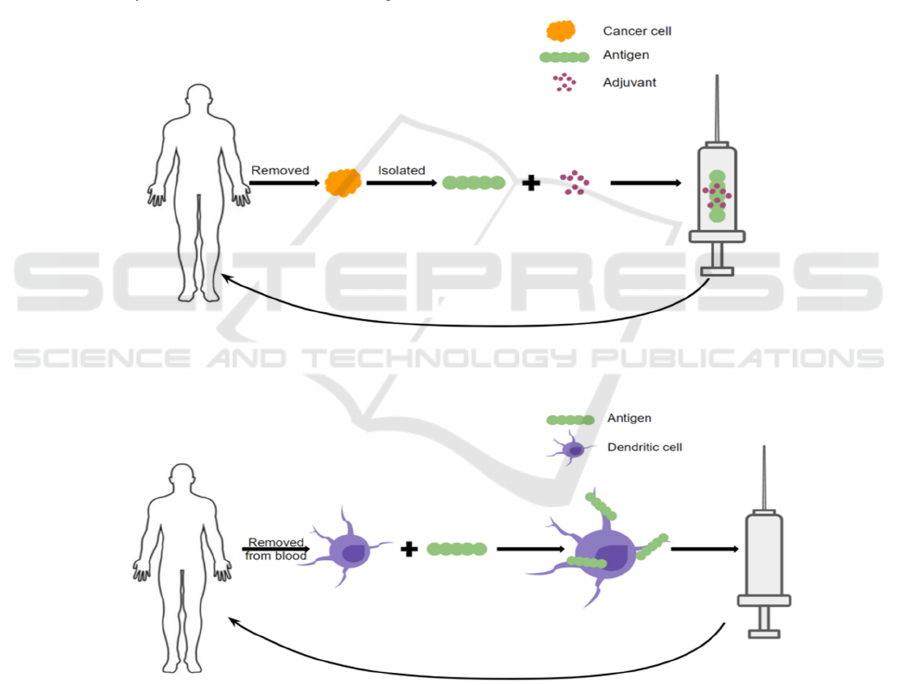
vaccination strategies (Melief et al, 2015) (Figure 1).
In one type, malignant cells are removed from a
patient’s tumor. Antigens will then be isolated and
mixed with an adjuvant and made into a vaccine given
back to the patient. By administering the cancer
antigen this way, the patient’s immune system is
primed to recognize the malignant cells as a threat and
thus eradicate the cancer. Another form of therapeutic
cancer vaccines involves removing the dendritic cells
(DCs) from the patient’s blood via leukapheresis and
loading them with cancer antigens ex vivo. Such
antigen-riched dendritic cells will then be infused
back into the patient and trigger an immune response
to the malignant cells (Vanderbilt, 2020) (Melief et
al, 2015). To date, three therapeutic cancer vaccines
have been approved in the United States: BCG for
treating early-stage bladder cancer, Provenge for
treating prostate cancer, and T-VEC for treating
melanoma (DeMaria and Bilusic, 2019).
Figure 1: Mechanisms of Therapeutic Cancer Vaccines (CRI, 2020).
This review elucidates the essential factors in
therapeutic cancer vaccine development including
target antigens and vaccine platforms. Tumor
vaccination targets can be subclassified as either
tumor-associated antigens (TAAs) or tumor-specific
antigens (TSAs), and depending on different causes
of cancer or location of tumor expression, the choice
of target antigens can be varied. Moreover,
therapeutic cancer vaccines can be developed via
different vaccine platforms including cellular
vaccines, viral vector-based vaccines, or molecular
vaccines, and each of the platforms contributes to the
development of therapeutic cancer vaccines uniquely.
Additionally, this review introduces four current FDA
ICBB 2022 - International Conference on Biotechnology and Biomedicine
378

approved therapeutic cancer vaccines including BCG,
Provenge, and T-VEC and provides a summary of
latest cancer vaccines in clinical trials againsting
different cancers including prostate cancer, breast
cancer, pancreatic cancer, colorectal cancer, renal cell
carcinoma, and hematological malignancies.
2 TARGET ANTIGENS
A wide variety of antigens expressed by tumor cells
can be targeted by therapeutic cancer vaccines
(DeMaria and Bilusic, 2019), yet the most imperative
factor when it comes to designing cancer vaccines is
the choice of antigen (Jou et al, 2021) with the ideal
antigens being expressed only by cancer cells,
presented on all cancer cells, highly immunogenic,
and indispensable for ensuring the survival of cancer
cells (Hollingsworth and Jansen, 2019). Tumor
vaccination targets are subclassified as either tumor-
associated antigens (TAAs) (Alatrash et al, 2019) or
tumor-specific antigens (TSAs) (Apavaloaei et al,
2020). Table 1 provides a summary of the differences
between TAAs and TSAs (Wang et al, 2019).
Table 1: TAAs VS. TSAs.
Tumor-associated
antigens (TAAs)
Tumor-specific
antigens (TSAs)
Expressed in
tumor cells?
Yes Yes
Expressed in
normal
cells?
Yes No
Most
common
cause
Post-translational
modifications/Genetic
amplification
Oncogenic driver
mutations that
produce novel
peptide sequences
Target types
Overexpressed
antigens
Differentiation
antigens
cancer/testis antigens
Oncogenic viral
antigens
Shared
neoantigens
Private
neoantigens
2.1 Tumor-Associated Antigens (TAAs)
TAAs are self-antigens expressed abnormally in
malignant cells and at low levels in normal cells
(Alatrash et al, 2019). TAAs target types include:
overexpressed antigens, cell lineage differentiation
antigens, and cancer/testis antigens (CT antigens)
(Table 1). Overexpressed antigens are a broad
category that encompasses any protein discovered in
higher concentrations in tumors than in healthy cells
and tissues (Bright et al, 2014). Examples of antigens
that are overexpressed in malignant cells include
MUC-1, mesothelin, HER2, hTERT. Cell lineage
differentiation antigens such as glycoprotein 100
(gp100), prostatic acid phosphatase (PAP), prostate-
specific antigen (PSA), and melanoma antigen
detected by T-cell 1 (MART-1) are generally not
expressed in adult tissue. CT antigens are usually only
seen in male germ cells, examples including human
melanoma antigen A1 (MAGE-A1), human
melanoma antigen A3 (MAGE-A3), and New York
esophageal carcinoma antigen 1 (NY-ESO-1)
(DeMaria and Bilusic, 2019) (Hollingsworth and
Jansen, 2019).
Several obstacles must be overcome in order to
develop effective therapeutic cancer vaccines against
TAAs. Foremost, growth and activation of self-
antigen-reactive T cells, particularly low-affinity T
cells, must be boosted through utilization of strong
adjuvants, co-stimulators, or repeated vaccination
because the high-affinity B cells and T cells for
recognizing these self-antigens may be inadequate to
trigger immune responses due to central and
peripheral tolerance mechanisms. Therefore,
therapeutic cancer vaccines utilizing TAAs must
elicit the remaining low affinity T cells for the
purpose of “breaking” the tolerance mechanisms (Jou
et al, 2021) (Hollingsworth and Jansen, 2019).
2.2 Tumor-Specific Antigens (TSAs)
TSAs are exclusively expressed by malignant cells,
and because of such tumor-specific properties, unlike
TAAs, TSAs are able to strongly trigger high-affinity
T cells and are less likely to be impacted by central
tolerance and autoimmunity. TSAs consist of
antigens expressed by neoantigens and oncoviruses
(Table 1) due to genetic modifications,
nonsynonymous mutations, or virally transmitted
genetic information in malignant cells (Jou et al,
2021).
Examples of oncogenic viral antigens include the
cervical cancer’s antigens, E7 and HPV E6. An
estimated 10-15 percent of human cancers worldwide
arise from viral infection (Liao, 2006), and such
highly immunogenic alien antigens are indeed the
cause of oncogenesis. Various highly effective
prophylactic antiviral vaccines have been developed
for preventing infections including HPV and HBV
vaccines; however, such vaccines have not proven to
be beneficial in the treatment of cancer that has
already developed, mainly due to the incapability of
humoral immunity to effectively eradicate vast
numbers of virus-infected malignant cells thus
Therapeutic Cancer Vaccines: Mechanism and Clinical Studies
379

requiring cell-mediated immune response
(Hollingsworth and Jansen, 2019).
Even if oncogenic viral antigens are unique to
specific tumor types, they are prevalent across many
patients. Comparably, some neoantigens may occur
in many tumor types and in many patients, hence the
so-called shared neoantigens; whereas, most
neoantigens are exclusively expressed in an
individual patient’s tumor, and they are referred to as
private neoantigens (Hollingsworth and Jansen,
2019). Thus, generation of personalized therapy is
essential for developing a cancer vaccine against an
individual patient’s private neoantigens. The process
of such personalized approach includes sequencing
the patient’s tumor genome, recognizing and
identifying the mutations, predicting neoantigens
utilizing computer-operated algorithms, developing a
personalized vaccine with the predicted neoantigen,
and eventually delivering the vaccine to the patient
(Kreiter et al, 2015).
3 VACCINE PLATFORMS
Anticancer therapy has been tested on a variety of
vaccine designs. These vaccines aim to induce
activation, proliferation, and maturation of T and B
cells by introducing tumor-associated peptides
complexed with major histocompatibility complex
(MHC) molecules to cognate receptors on T and B
cells. Antitumor immune responses are effective with
the present of T cells as tumor antigens are often
generated from intracellular proteins. Improved
understanding of T cell activation and activity has
aided recent developments in therapeutic cancer
vaccine innovations (Hollingsworth and Jansen,
2019). Generally, platforms of therapeutic cancer
vaccines are classified as: cellular vaccines, viral
vector-based vaccines, and molecular vaccines
(Peptide, DNA, or RNA) (Jou et al, 2021).
3.1 Cellular Vaccines
Cellular vaccines refers to cell-based vaccines that are
developed utilizing autologous patient-derived tumor
cells or allogeneic tumor cell line-derived cells (Jou
et al, 2021) (Le et al, 2010) and can be administered
to patients utilizing cell lysates or irradiated whole-
tumor cells (Srivatsan et al, 2014). GVAX vaccine is
an example of cellular vaccine utilizing genetically
modified whole-tumor cells (Jou et al, 2021) to
secrete Granulocyte-macrophage colony-stimulating
factor (GM-CSF), which is a cytokine that boost the
activation of dendritic cells and facilitates antigen
presentation to both T and B cells thus stimulating the
immune system against malignant cells (Nemunaitis,
2005). GVMAX vaccines have shown promising
efficacy in stimulating immune system responses and
inducing tumor regression, yet several clinical studies
demonstrate that GVAX vaccines have limited
effectiveness in prostate cancer, pancreatic cancer,
lung cancer, and melanoma (Hollingsworth and
Jansen, 2019). Autologous dendritic cells (DCs) are
also utilized for cellular cancer vaccine development
as they act as tumor antigen consumers, processors,
and presenters (Jou et al, 2021), and they are either
pulsed with peptide antigen or infected with a viral
vector. Sipuleucel-T (Provenge) is a conventional
cancer vaccine for treating metastatic castration-
resistant prostate cancer (mCRPC) (DeMaria and
Bilusic, 2019). Researchers are currently developing
and inspecting other DC vaccines with one instance
being the adenovirus MART-1-engineered
autologous DC vaccine for treating metastatic
melanoma (Butterfield et al, 2008). Other cellular
vaccines utilized microorganisms to deliver tumor
antigens and elicit immune responses (Jou et al, 2021)
(DeMaria and Bilusic, 2019) (Hollingsworth and
Jansen, 2019).
3.2 Viral Vector-Based Vaccines
Several viruses have been utilized to develop
therapeutic cancer vaccines. Such viral vector-based
vaccines exploit genetically modified versions of
different viruses as vectors and are constructed to
eliminate and replicate within malignant cells (Jou et
al, 2021). An advantage for viral vector-based
vaccines is that the patient’s immune system is able
to respond and recognize viruses efficiently, with
both adaptive and innate immune systems
collaborating to produce robust and substantial
responses. Pattern recognition receptors (PRRs) will
activate antigen-presenting cells in response to viral
pathogen-associated molecular patterns
(Hollingsworth and Jansen, 2019). However, repeat
vaccination will be limited due to the fact that such
viral vectors are neutralized by antiviral immune
response. A common approach to address this
advantage is the application of a heterologous prime-
boost strategy in which one virus vector delivers a
tumor antigen first, followed by a boost with the same
tumor antigen carried by a different viral vector (Pan
et al, 2020).
ICBB 2022 - International Conference on Biotechnology and Biomedicine
380
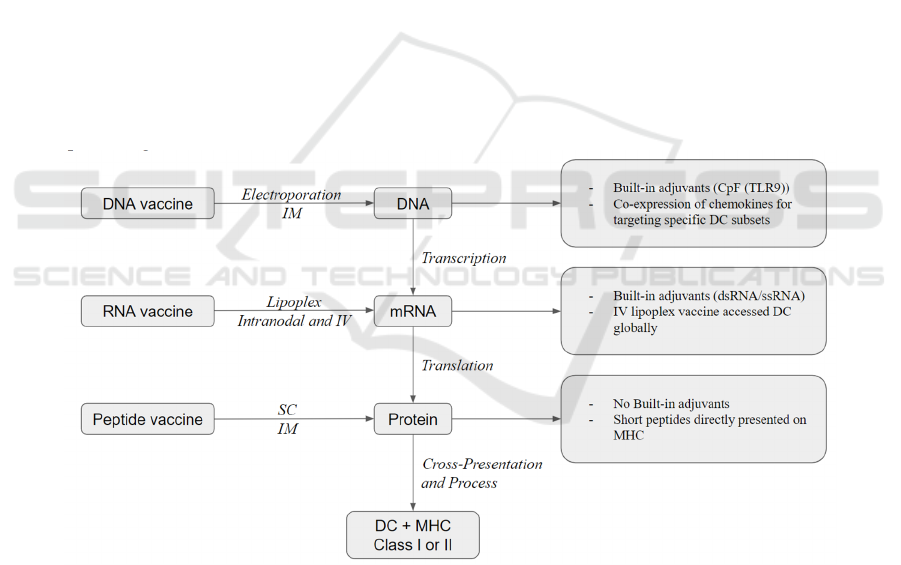
3.3 Molecular Vaccines
There are three types of molecular vaccines based on
the source of tumor antigen delivery: peptide, DNA,
and RNA. And each of these three vaccine platforms
have the ability to elicit significant T cell responses as
well as therapeutic effects against established
diseases (Li et al, 2014). Figure 2 provides a
simplified illustration of the three different
therapeutic cancer vaccine delivery types.
Peptide-based vaccines, also known as synthetic
long peptide (SLP) vaccines, relies on usage of exact
MHC class I-binding short peptide fragments to
design the elicitation of high targeted immunological
responses thus avoiding reactogenic sequences
(Bijker et al, 2008). However, single antigen-based
short peptides may fail to overcome loss of antigen
expression within the tumor or fail to encode
sufficient antigenic material to stimulate potent
immune responses, thus immune adjuvant is usually
required for developing peptide vaccines (DeMaria
and Bilusic, 2019). Short peptides, usually less than
fifteen amino acids, can bind toMHC class I
molecules and do not necessitate processing by
antigen-presenting cells. Nevertheless, T cell
dysfunction and tolerogenic signal might occur if
such short peptides bind to other cells that do not
provide correct co-stimulation (Overwijk, 2017).
Moreover, C4 helper T cells will not be activated by
short peptides. Researchers have put effort into
improving peptide vaccines’ quality by utilizing
amphiphilic peptides and combining them with other
immune modulators (Lysén et al, 2020).
DNA vaccines have built-in adjuvants such as
CpG (TLR9) (Li et al, 2014) and represent a
condensed conformation of TAAs, yet they require
additional transcriptional and translational steps
before being cross-represented on DCs (Sahin et al,
2017). Furthermore, DNA vaccines can be
electroporated at the injection location directly. When
administered at high dosages by intramuscular
injection combined with electroporation, DNA
vaccines are the most efficient in generating sufficient
antigen processing and presentation to induce CD8+
T and CD4+T responses (Li et al, 2014). A further
innovation of CD8+ T cell induction is a technique in
neoepitope-specific vaccines in which antibody
response and T cells are boosted due to co-expression
of DNA and chemokines for targeting specific
dendritic cell subsets (Ewer et al, 2016).
Figure 2: Simplified illustration of three therapeutic cancer vaccine delivery types (Li et al, 2014).
Similar to DNA vaccines, RNA vaccines have
built-in adjuvants such as dsRNA and ssRNA.
Nonetheless, in contrast to DNA vaccines, RNA
vaccines do not require an extra transcriptional
process. Thus, they are closer to protein antigen
expression and presentation on MHC molecules (Li
et al, 2014). RNA vaccines can be injected into lymph
nodes directly or intravenously injected with the help
of recently created nanoparticles (lipoplexes)
(Fletcher, 2019). The majority of RNA vaccines in
clinical studies nowadays have utilized mRNA while
the use of RNA replicon is also being investigated
(Plosker, 2012).
Therapeutic Cancer Vaccines: Mechanism and Clinical Studies
381

4 APPROVED THERAPEUTIC
CANCER VACCINES
To date, only three therapeutic cancer vaccines have
been approved and utilized in the United States: BCG
for treating early-stage bladder cancer, Provenge for
treating prostate cancer, and T-VEC for treating
melanoma (DeMaria and Bilusic, 2019).
4.1 BCG (Baciile Calmette-Guerin;
Sanofi Pasteur)
BCG live, also known as TheraCys and TICE, was
approved by the FDA in 1990 as a cancer
immunotherapy for the treatment of early-stage
bladder cancer. Concretely, it is developed for
intravesical utilization for treating and preventing
urothelial carcinoma in situ of the urinary bladder and
primary Ta or T1 urothelial carcinoma after
transurethral resection (DeMaria and Bilusic, 2019).
Healthcare professionals will administer BCG as a
liquid drug into the patient’s bladder via a catheter.
Such an approach enables direct contact of the drug
and the cancer cells in the bladder thus ensuring the
right target for the patient’s immune system.
However, BCG immunotherapy can cause various
side effects which include urinary tract infection,
blood in the urine, discomfort in the bladder, etc
(Anassi and Ndefo, 2011). According to research, in
patients with superficial bladder cancer who undergo
maintenance treatment, intravesical BCG remarkably
lowers the likelihood of progression following
transurethral resection (DeMaria and Bilusic, 2019).
4.2 Provenge (Sipuleucel-T; Dendreon
Corporation)
Provenge, also known as Sipuleucel-T, was approved
by the FDA in 2010 as an autologous cellular
immunotherapy for treating metastatic castration-
resistant prostate cancer (mCRPC), which is a form
of advanced prostate cancer (DeMaria and Bilusic,
2019). Provenge is developed to activate T cell
response against prostatic acid phosphatase (PAP),
which is an antigen expressed in most prostate
cancers yet not in non-prostate tissues. It is an
effective personalized vaccine therapy in which each
treatment dose is designed specifically for each
individual patient utilizing the patient’s own immune
system. Patient’s immune cells, concretely
autologous peripheral blood mononuclear cells, will
be collected through leukapheresis, and these immune
cells will be combined with a protein (Conry et al,
2018) that triggers the immune cells to detect prostate
cancer. Provenge only has mild to moderate side
effects that usually last one to two days. Researches
have shown with the help of provenge, men with
prostate cancers are able to live longer, and the risk of
death due to prostate cancer has been reduced (The
ASCO Post, 2019).
4.3 T-VEC (talimogene laherparepvec;
Amgen)
Imlygic, also known as talimogene laherparepvec or
T-VEC, was approved by the FDA in 2015 for treating
advanced melanoma. It is also the first oncolytic viral
therapy in the United States (DeMaria and Bilusic,
2019) in which an oncolytic herpes virus, a virus that
only infects cancer cells, is utilized. Such oncolytic
virus is genetically modified to ensure its replication
within tumors and production of the granulocyte-
macrophage colony stimulating factor (GM-CSF).
Usually, talimogene laherparepve is injected directly
into the detected lymph nodes, subcutaneous lesions,
or skin lesions that surgery cannot remove.
Talimogene laherparepvec injected into the tumor
will break down the tumor cells and release tumor-
derived antigens. Such tumor-derived antigens
together with GM-CSF can stimulate the immune
response against the tumor. Side effects of this
therapy include fatigue, chills, fever, nausea,
vomiting, diarrhea, constipation, abdominal pain,
dizziness, injection site pain or inflammation, etc
(Melero et al, 2014). According to research,
talimogene laherparepvec has effectively treated
approximately 40% of patients with surgically
unremovable tumors (Geary and Salem, 2013).
5 CURRENT THERAPEUTIC
CANCER VACCINES
UNDERGO CLINICAL TRIALS
Researchers are currently investigating more possible
therapeutic cancer vaccines for different types of
cancer which include prostate cancer, breast cancer,
pancreatic cancer, colorectal cancer, renal cell
carcinoma, hematological malignancies, etc
(Wittendorf et al, 2012).
As aforementioned, Provenge (sipuleucel-T) is a
currently utilized therapeutic cancer vaccine for
treating patients with spreaded prostate cancer.
Recent studies are investigating and developing
therapeutic cancer vaccines for early-stage prostate
cancer treatments (Wittendorf et al, 2012) (Middleton
ICBB 2022 - International Conference on Biotechnology and Biomedicine
382

et al, 2013). Current clinical trials are investigating
HER2-derived peptides along with additional
immunostimulatory agents including Granulocyte-
macrophage colony-stimulating factor (GM-CSF),
cyclophosphamide, or poly-ICLC. Research has
shown that directly against HER2-derived peptides
via active immunotherapy have beneficial effects for
women with breast tumors with low level HER2
(Wittendorf et al, 2012) (Cancer.NET, 2020).
Telomerase peptide vaccine GV1001 and the
allogeneic tumor-cell vaccine algenpantucel-L are the
most commonly investigated cancer
immunotherapies for pancreatic cancer treatment.
Several phase III and II studies had promising early-
phase trials yet failed in the later stage trials (Amin et
al, 2013).
Current clinical studies on colorectal cancer
treatments involves developing therapeutic cancer
vaccines to educate the immune system to combat
cells with antigens include carcinoembryonic antigen
(CEA), MUC1, guanylyl cyclase C, and NY-ESO-1
(Wittendorf et al, 2012) (Keiholz et al, 2009). AGS-
003 is a current immunotherapy development for
treating renal cell carcinoma (RCC), it is a
personalized therapy utilizing DCs transfected with
patient-specific cancer cell RNA and a truncated,
synthetic human CD40 ligand (Wittendorf et al,
2012).Current clinical studies on hematological
malignancies treatments involve developing
therapeutic cancer vaccines to educate the immune
system to combat cells with antigens include WT1,
MAGE, MUC1 and the preferentially expressed
antigen of melanoma (PRAME) (Wittendorf et al,
2012).
6 CONCLUSION
The development of therapeutic cancer vaccines have
improved the field of cancer immunotherapy
tremendously. As a result of the development of three
FDA approved therapeutic cancer vaccines, which
include BCG, Provenge, and T-VEC, our
understanding of the tumor biology as well as
pathways of the immune system have reached the
next level.
Despite the fact that therapeutic cancer vaccine
development has had promising achievement and
considerable results in most phase I and some phase
II clinical trials, the vast majority of therapeutic
cancer vaccines have failed to exhibit clinical
improvements in phase III trials over the past years
due to low immunogenicity of tumor antigens,
difficulty in targeting established tumor, and
immunosuppressive tumor microenvironment. Thus,
testing therapeutic cancer vaccines in early stages of
cancer to minimize the disease burden and immune
tolerance should be prioritized. Moreover, it’s
essential to establish better strategies of incorporating
therapeutic cancer vaccines into the standard
treatment such that immune tolerance is less
formidable or combining therapeutic cancer vaccines
with other cancer treatment therapies thus promoting
synergistic treatment effects.
Developing personalized therapeutic cancer
vaccines is a difficult task overall. Fortunately,
researchers are still indefatigably investigating
potential solutions to the aforementioned challenges
and examining more therapeutic cancer vaccines in
clinical trials. It is undoubtedly that more therapeutic
cancer vaccines can be approved in the future.
REFERENCES
Alatrash, G., Crain, A. K., & Molldrem, J. J. (2019).
Chapter 7 - Tumor-Associated Antigens. In G. Socié.,
R. Zeiser, & B. R. Blazar (Eds.), Immune Biology of
Allogeneic Hematopoietic Stem Cell Transplantation
(Second Edition, pp.107-125). Academic Press.
Apavaloaei, Hardy, M.-P., Thibault, P., & Perreault, C.
(2020). The origin and immune recognition of tumor-
specific antigens. Cancers, 12(9), 1–13.
Anassi, & Ndefo, U. A. (2011). Sipuleucel-T (Provenge)
injection the first immunotherapy agent (Vaccine) for
hormone-refractory prostate cancer. P&T
(Lawrenceville, N.J.), 36(4), 197–202.
Amin, Dudek, A., Logan, T., Lance, R. S., Holzbeierlein, J.
M., Master, V. A., Pal, S. K., Knox, J. J., Karsh, L. I.,
Plessinger, D., Nicolette, C. A., & Figlin, R. A. (2013).
Prolonged survival with personalized immunotherapy
(AGS-003) in combination with sunitinib in
unfavorable risk metastatic RCC (mRCC). Journal of
Clinical Oncology, 31(6_suppl), 357–357.
Butterfield, Comin-Anduix, B., Vujanovic, L., Lee, Y.,
Dissette, V. B., Yang, J.-Q., Vu, H. T., Seja, E.,
Oseguera, D. K., Potter, D. M., Glaspy, J. A.,
Economou, J. S., & Ribas, A. (2008). Adenovirus
MART-1-engineered autologous dendritic cell vaccine
for metastatic melanoma. Journal of Immunotherapy
(1997), 31(3), 294–309.
Bijker, van den Eeden, S. J. F., Franken, K. L., Melief, C.
J. M., van der Burg, S. H., & Offringa, R. (2008).
Superior induction of anti‐tumor CTL immunity by
extended peptide vaccines involves prolonged, DC‐
focused antigen presentation. European Journal of
Immunology, 38(4), 1033–1042.
Bright, Bright, J. D., & Byrne, J. A. (2014). Overexpressed
oncogenic tumor-self antigens. Human Vaccines &
Immunotherapeutics, 10(11), 3297–3305.
Cancer.Net (2020 August). What are Cancer Vaccines?
Therapeutic Cancer Vaccines: Mechanism and Clinical Studies
383

Cancer Research Institute. (2020, October). What is Cancer
Immunotherapy? UC San Diego Health.
https://www.cancerresearch.org/en-
us/immunotherapy/what-is-immunotherapy
Conry, Westbrook, B., McKee, S., & Norwood, T. G.
(2018). Talimogene laherparepvec: First in class
oncolytic virotherapy. Human Vaccines &
Immunotherapeutics, 14(4), 839–846.
DeMaria, & Bilusic, M. (2019). Cancer Vaccines.
Hematology/oncology Clinics of North America, 33(2),
199–214.
Ewer, Lambe, T., Rollier, C. S., Spencer, A. J., Hill, A. V.,
& Dorrell, L. (2016). Viral vectors as vaccine
platforms: from immunogenicity to impact. Current
Opinion in Immunology, 41, 47–54.
Fletcher, J. (2019, February 6). Bladder cancer: What to
know about BCG treatment. MedicalNewsToday.
https://www.medicalnewstoday.com/articles/324385
Geary, & Salem, A. K. (2013). Prostate cancer vaccines:
Update on clinical development. Oncoimmunology,
2(5), e24523–e24523.
Hollingsworth, & Jansen, K. (2019). Turning the corner on
therapeutic cancer vaccines. Npj Vaccines, 4(1), 7–7.
Jou, Harrington, K. J., Zocca, M.-B., Ehrnrooth, E., &
Cohen, E. E.. (2021). The changing landscape of
therapeutic cancer vaccines-novel platforms and
neoantigen identification. Clinical Cancer Research,
27(3), 689–703.
Kreiter, S., Vormehr, M., van de Roemer, N. et al. Mutant
MHC class II epitopes drive therapeutic immune
responses to cancer. Nature 520, 692–696 (2015).
Keilholz, Letsch, A., Busse, A., Asemissen, A. M., Bauer,
S., Blau, I. W., Hofmann, W.-K., Uharek, L., Thiel, E.,
& Scheibenbogen, C. (2009). A clinical and
immunologic phase 2 trial of Wilms tumor gene
product 1 (WT1) peptide vaccination in patients with
AML and MDS. Blood, 113(26), 6541–6548.
Le, Pardoll, D. M., & Jaffee, E. M. (2010). Cellular vaccine
approaches. The Cancer Journal (Sudbury, Mass.),
16(4), 304–310.
Liao. (2006). Viruses and human cancer. The Yale Journal
of Biology & Medicine, 79(3-4), 115–122.
Li, Joshi, M. D., Singhania, S., Ramsey, K. H., & Murthy,
A. K. (2014). Peptide vaccine: Progress and challenges.
Vaccines (Basel), 2(3), 515–536.
Lysén, Braathen, R., Gudjonsson, A., Tesfaye, D. Y.,
Bogen, B., & Fossum, E. (2020). Author Correction:
Dendritic cell targeted Ccl3- and Xcl1-fusion DNA
vaccines differ in induced immune responses and
optimal delivery site. Scientific Reports, 10(1), 5944–
5944.
Melief, Van Hall, T., Arens, R., Ossendorp, F., & Van Der
Burg, S. H. (2015). Therapeutic cancer vaccines. The
Journal of Clinical Investigation, 125(9), 3401–3412.
Melero, Gaudernack, G., Gerritsen, W. ., Huber, C.,
Parmiani, G., Scholl, S., Thatcher, N., Wagstaff, J.,
Zielinski, C., Faulkner, I., & Mellstedt, H. (2014).
Therapeutic vaccines for cancer: an overview of clinical
trials. Nature Reviews. Clinical Oncology, 11(9), 509–
524.
Mittendorf, Clifton, G. T., Holmes, J. P., Clive, K. S., Patil,
R., Benavides, L. C., Gates, J. D., Sears, A. K.,
Stojadinovic, A., Ponniah, S., & Peoples, G. E. (2012).
Clinical trial results of the HER-2/neu (E75) vaccine to
prevent breast cancer recurrence in high-risk patients:
From US Military Cancer Institute Clinical Trials
Group Study I-01 and I-02. Cancer, 118(10), 2594–
2602.
Middleton, Valle, J. W., Wadsley, J., Propper, D., Coxon,
F. Y., Ross, P. J., Madhusudan, S., Roques, T.,
Cunningham, D., Corrie, P., Greenhalf, W., Shaw, V.,
Cox, T. F., Silcocks, P., Nanson, G., & Neoptolemos, J.
P. (2013). A phase III randomized trial of
chemoimmunotherapy comprising gemcitabine and
capecitabine with or without telomerase vaccine
GV1001 in patients with locally advanced or metastatic
pancreatic cancer. Journal of Clinical Oncology,
31(18_suppl), LBA4004–LBA4004.
Nemunaitis. (2005). Vaccines in cancer: GVAX®, a GM-
CSF gene vaccine. Expert Review of Vaccines, 4(3),
259–274.
Overwijk. (2017). Cancer vaccines in the era of checkpoint
blockade: the magic is in the adjuvant. Current Opinion
in Immunology, 47, 103–109. Alloatti, Kotsias, F.,
Magalhaes, J. G., & Amigorena, S. (2016). Dendritic
cell maturation and cross-presentation: timing matters.
Immunological Reviews, 272(1), 97–108.
Pan, Jia, R., Li, J., Wang, M., Chen, S., Liu, M., Zhu, D.,
Zhao, X., Wu, Y., Yang, Q., Yin, Z., Jing, B., Huang,
J., Zhang, S., Zhang, L., Liu, Y., Yu, Y., Tian, B., Pan,
L., … Cheng, A. (2020). Heterologous prime-boost: an
important candidate immunization strategy against
Tembusu virus. Virology Journal, 17(1), 67–67.
Saxena, & Bhardwaj, N. (2017). Turbocharging
vaccines: emerging adjuvants for dendritic cell based
therapeutic cancer vaccines. Current Opinion in
Immunology, 47, 35–43.
Plosker. (2012). Sipuleucel-T: In Metastatic Castration-
Resistant Prostate Cancer. Drugs (New York, N.Y.),
71(1), 101–108.
Romei, Ciampi, R., & Elisei, R. (2016). A comprehensive
overview of the role of the RET proto-oncogene in
thyroid carcinoma. Nature Reviews. Endocrinology,
12(4), 192–202.
Ronen, A., & Glickman, B. W. (2001). Human DNA repair
genes. Environmental and molecular mutagenesis,
37(3), 241–283.
Srivatsan, Patel, J. M., Bozeman, E. N., Imasuen, I. E., He,
S., Daniels, D., & Selvaraj, P. (2014). Allogeneic tumor
cell vaccines: The promise and limitations in clinical
trials. Human Vaccines & Immunotherapeutics, 10(1),
52–63.
Sahin, Derhovanessian, E., Miller, M., Kloke, B.-P., Simon,
P., Löwer, M., Bukur, V., Tadmor, A. D.,
Luxemburger, U., Schrörs, B., Omokoko, T., Vormehr,
M., Albrecht, C., Paruzynski, A., Kuhn, A. N., Buck, J.,
Heesch, S., Schreeb, K. H., Müller, F., … Türeci, Ö.
(2017). Personalized RNA mutanome vaccines
mobilize poly-specific therapeutic immunity against
cancer. Nature (London), 547(7662), 222–226.
ICBB 2022 - International Conference on Biotechnology and Biomedicine
384

The ASCO Post. (2019, January 1). How Effective Is
Talimogene Laherparepvec Injection in Metastatic
Melanoma?
Vanderbilt University Medical Center (2020, December 7).
Cancer Vaccines: Are We There Yet? Vanderbilt
University.
Wang, Wu, C.-F., Rajasekaran, N., & Shin, Y. K. (2019).
Loss of Tumor Suppressor Gene Function in Human
Cancer: An Overview. Cellular Physiology and
Biochemistry, 51(6), 2647–2693.
World Health Organization. (2021, September 21).
“Cancer.” Retrieved from https://www.who.int/news-
room/fact-sheets/detail/cancer
Therapeutic Cancer Vaccines: Mechanism and Clinical Studies
385
