The History of Urea and Its Use in the Modern Fertilizer Industry
Iris Yan Wong
Deerfield Academy, Massachusetts, 01342-0087, U.S.A.
Keywords:
Synthetic Urea, Isomerization Reaction, Haber-Bosch Process, Industrial Process.
Abstract: From the common name of the organic compound “urea”, it can be inferred that urea is a major component
of urine. Urea naturally occurs in most mammals and is crucial for removing toxic waste products from the
human body. This work investigates the history of urea, including the discovery of the “urea cycle” in humans.
Moreover, urea was the first organic compound to be synthesized from inorganic compounds, bringing about
a new definition of organic chemistry. This work examines the specific mechanism behind Fredrich Wohler’s
synthesis of urea from inorganic compounds. Furthermore, urea is now synthesized on a large scale for
nitrogen-based crop fertilizers, and such modern processes have great implications on the environment.
1
INTRODUCTION
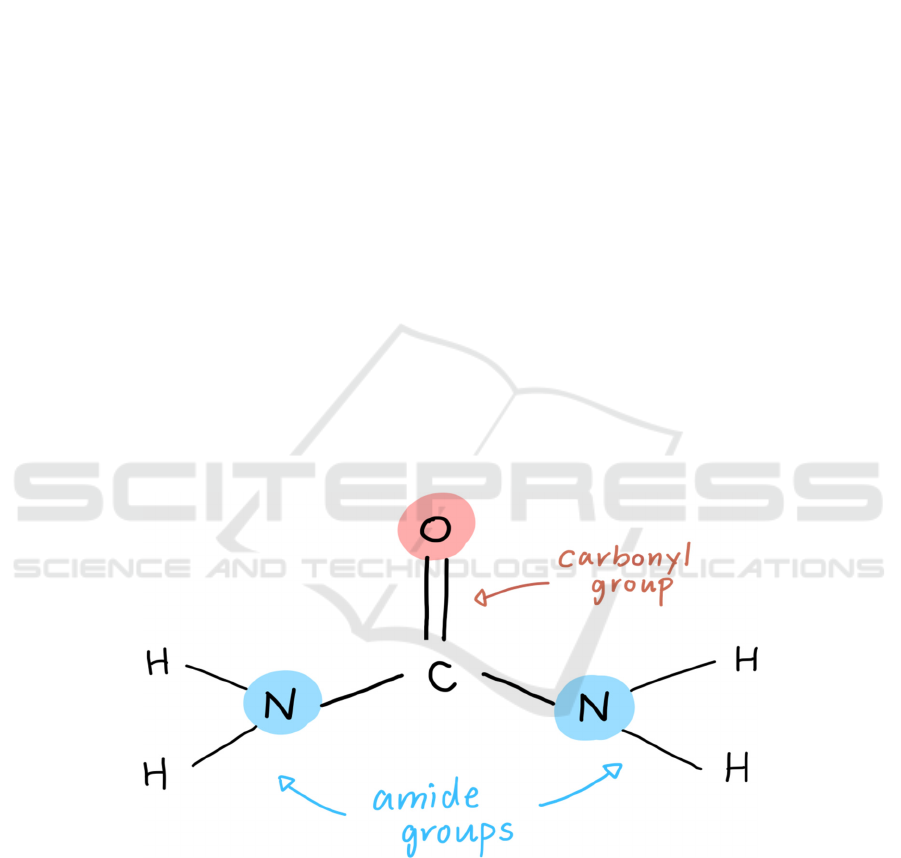
Urea is composed of a carbamide--- a carbonyl group
attached to two amide groups (see Figure 1). Its
molecular formula is CH
4
N
2
O, and its exact mass is
60.06g. Due to the carbonyl group in the compound,
urea is polar. Furthermore, it is highly soluble in
water and has a neutral charge overall (National
Center for Biotechnology Information, 2021).
Figure 1: Urea Molecular Diagram.
2
HISTORY OF UREA
DISCOVERIES
The first known description of urea is by Belgian
chemist Jean Batiste von Helmont in 1664, who
realised that there was a natural salt in urine (Raine,
1973). In 1732, Dutch chemist Hermann Boerhaave
published his chemistry textbook Ementa Chemiae,
which included the purification method of urea from
urine (Raine, 1973). Almost 50 years after
Boerhaave, French chemist Hilaire Rouelle found the
same method as his (Eknoyan, 2017). Despite
Boerhaave’s earlier work, Rouelle is frequently
attributed for the discovery and isolation of urea from
urine (Raine, 1973).
Wong, I.
The History of Urea and Its Use in the Modern Fertilizer Industry.
DOI: 10.5220/0012032100003633
In Proceedings of the 4th International Conference on Biotechnology and Biomedicine (ICBB 2022), pages 435-439
ISBN: 978-989-758-637-8
Copyright
c
2023 by SCITEPRESS – Science and Technology Publications, Lda. Under CC license (CC BY-NC-ND 4.0)
435
2.1
Wöhler’s Synthesis of Urea from
Inorganic Compounds
Nearly 100 years after Boerhaave’s findings were
published, German chemist Fredrich Wöhler made
the groundbreaking achievement of synthesizing urea
in a lab. Previously, scientists had believed that
naturally occurring organic compounds only
originated from living organisms from other organic
compounds (Rabinovich, 2007). This idea, known as
the “vital force theory”, expresses that a hypothetical
“vital force” in living organisms was necessary for
the formation of an organic compound (Friedmann,
1997). Upon this discovery, Wöhler wrote to his
mentor, “I can no longer, as it were, hold back my
chemical urine; and I have to let out that I can make
urea without needing a kidney, whether of man or
dog.” (Friedmann, 1997). The popularity of the “vital
force theory” declined as Wöhler’s synthesis put an
emphasis on chemical structures in compounds. Since
Wöhler’s discovery almost two centuries ago,
thorough studies continue to be conducted on
structural chemistry to further understand the
intricacy of organic compounds (Heitmann, 1989).
Wöhler’s original intention had not been to create
synthetic urea. Rather, this unanticipated discovery
came when he combined lead cyanate with ammonia
and water to form ammonium cyanate and lead
hydroxide.
Pb(OCN)
2
+ 2NH
3
+ 2H
2
O → Pb(OH)
2
+
2NH
4
(OCN)
Wöhler’s original experiment (Carr, 2018)
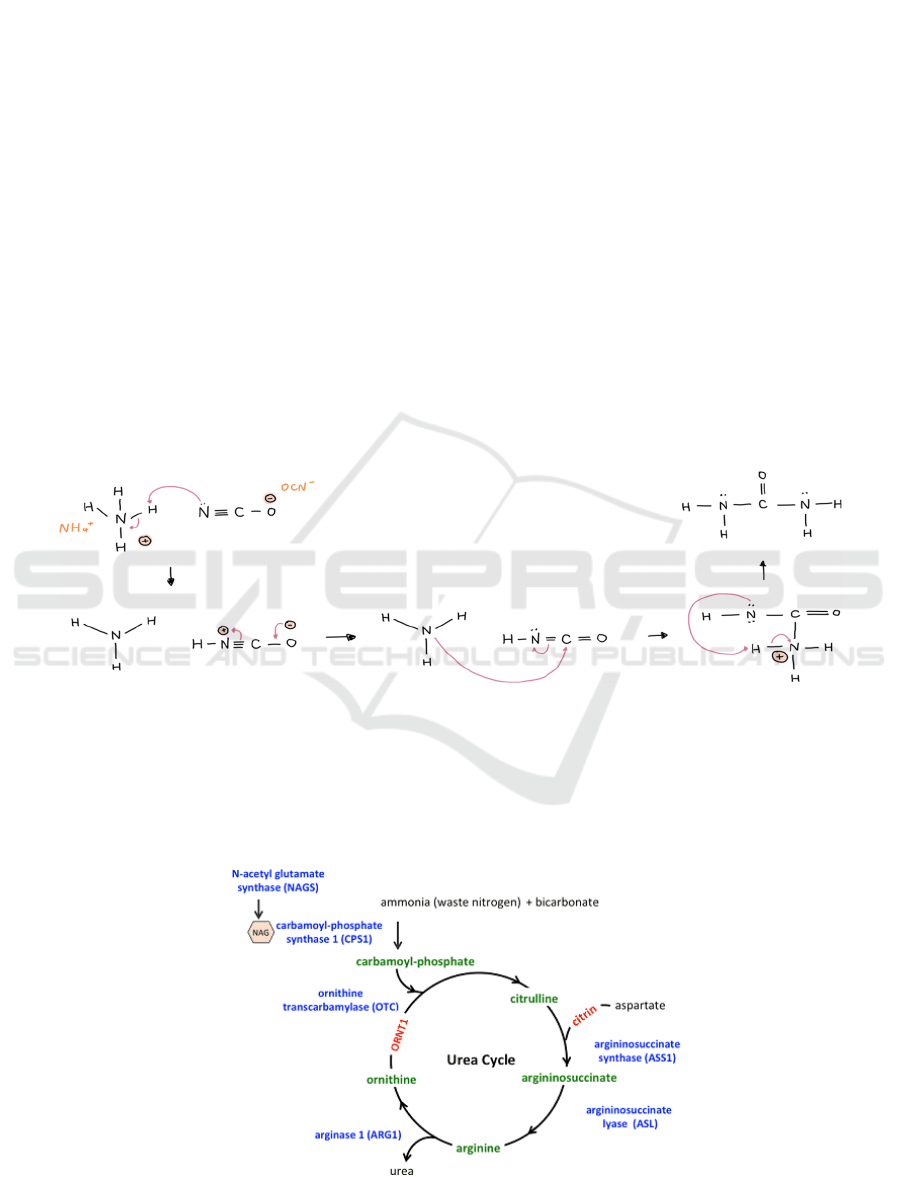
When Wöhler heated ammonium cyanate, he
realised that its properties aligned entirely with the
properties of urea. This, as he later found, was due to
an isomerization that had taken place after the
ammonium cyanate was formed. As Figure 2 shows,
the cyanate was the nucleophile that attacked the
hydrogen attached to ammonium. This resulted in
ammonium cyanate decomposing into ammonia and
cyanic acid and forming a reactive carbonyl. From
there, the ammonia attacked the carbonyl, leading to
the final planar urea molecule after proton transfers.
Figure 2: Reversible isomerization reaction from ammonium cyanate into urea.
2.2 Discovery of the Human Urea Cycle
This synthesis opened up a new possibility for
organic chemistry: the mass production of organic
materials. Scientists worked to find ways to
synthesize organic compounds from inorganic ones.
Amongst these included new methods of synthesizing
urea from other compounds. Further research was
also conducted on naturally occurring urea.
Figure 3: Overview of Urea Cycle (Ah Mew, 2003).
ICBB 2022 - International Conference on Biotechnology and Biomedicine
436
In 1932, Hans Krebs and Kurt Heinselet
discovered the natural “urea cycle” in the human
body (see Figure 3). It was the first metabolic cycle to
be proposed, and it details the formation and
excretion of urea. When toxic ammonia is naturally
formed in digestive processes, the body relies on the
cycle to remove it safely. Specifically, ammonia is
converted into urea in the liver’s mitochondria and
cytoplasm, with the help of enzyme catalysts like
ornithine transcarbamylase and argininosuccinate
synthetase (Barmore, 2021).
2NH
3
+ CO
2
+ 3ATP → H
2
N(CO)NH
2
+ H
2
O +
3ADP
Overall reaction from ammonia to urea
(Cheriyedath, 2019)
3 MODERN PROCESSES OF
SYNTHESIZING UREA AND
ITS IMPLICATIONS
On the other hand, synthetic urea is found in various
products, such as instant cold packs, skin care
products and resins, but it is most used in nitrogen-
based fertilizers. This is because urea has the highest
nitrogen content amongst solid nitrogen fertilizers
(Bradley, 2018). In fact, due to its properties, more
than 90% of urea production is used for agricultural
purposes (American Chemical Society, 2021).
3.1 Employment of Haber-Bosch
Process to Synthesize Urea from
Ammonia
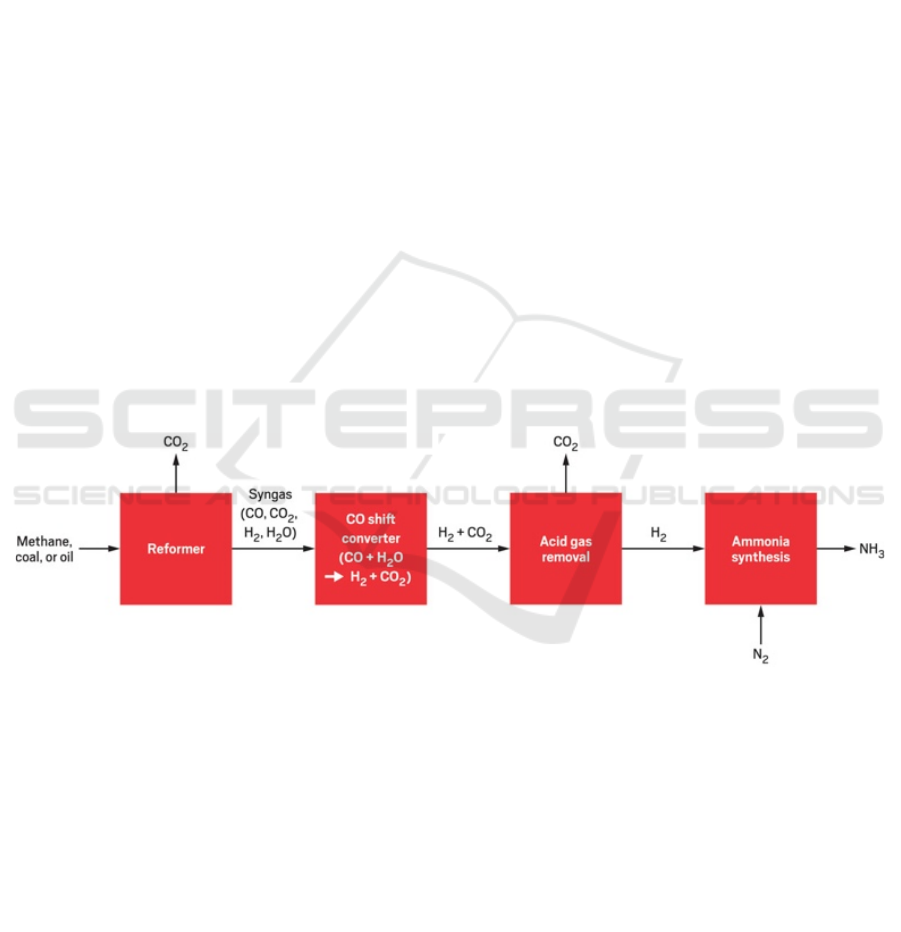
To facilitate global production of urea, a specific
process known as the Haber-Bosch process is
employed. As seen in Figure 4, hydrogen is first
formed by reacting methane or other natural gases
with steam. Under high temperatures, the hydrogen is
synthesized with gaseous nitrogen to make ammonia.
Ammonia is subsequently combined with carbon
dioxide to form ammonium carbamate, which then
decomposes into urea and water (Copplestone, 2017).
Though this synthesis equation was always known,
German chemists Fritz Haber and Carl Bosch found
ideal conditions for it to happen with much higher
yield than before: high temperatures, high pressures
and typically an iron-based catalyst. The process also
removes substances such as carbon monoxide, water,
and other carbon oxides during synthesis
(Copplestone, 2017). This developed industrial
process is key to the global output of 220 million tons
of urea per year (American Chemical Society, 2021).
Figure 4: Overview of the Haber-Basch process (Boerner, 2019).
CO
+ H
2
O → CO
2
+ H
2
Hydrogen synthesis in industrial processes
(Copplestone, 2017)
N
2
+ 3H
2
⇌ 2NH
3
Ammonia synthesis in industrial processes
(Copplestone, 2017)
2NH
3
+ CO
2
⇌ NH
2
COONH
4
(ammonium
carbamate)
NH
2
COONH
4
⇌ H
2
O + NH
2
CONH
2
(urea)
Urea synthesis in industrial processes
(Copplestone, 2017)
3.2 Existing Issues with Mass
Production of Synthetic Urea and a
Possible New Method
Such a wide-scale level of production comes at a
global cost, though. The multitude of problems begins
from the compound itself and extends all the way to
its industrial process. For instance, Christer Aakeröy
from Kansas State University explains that urea is
highly soluble in water, offering an unfavorable
property for storage and transport (Sandhu, 2018).
Farmers therefore tend to overuse urea fertilizer to
compensate for potential losses, leading to
The History of Urea and Its Use in the Modern Fertilizer Industry
437
inefficiencies and increased nitrogen concentration in
the air (Bradley, 2018). The most pressing issue,
however, is the copious amounts of energy needed to
produce urea. 80% of ammonia produced globally is
used specifically for urea synthesis (Chen, 2020), yet
ammonia production through the Haber-Bosch
process accounts for 1-2% of worldwide energy
consumption and 1.44% of CO
2
emissions (Kyriakou,
2020). This makes it the industrial process that emits
the most CO
2
worldwide (American Chemical
Society, 2021).
The harsh impacts of the process on the
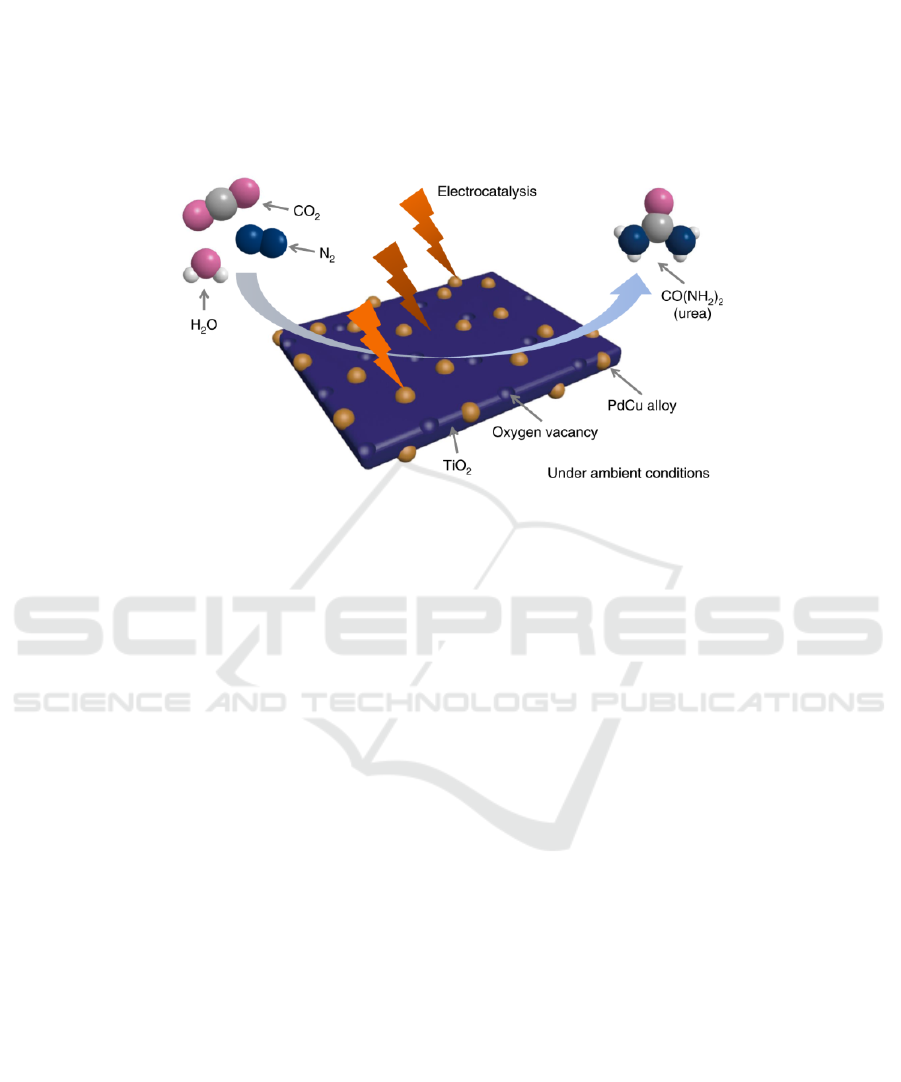
environment have propelled scientists to investigate
more energy-efficient methods of urea synthesis.
Figure 5: Electrocatalysis to synthesize urea (Chen, 2020).
In 2018, chemical engineer Shaungyin Wang and
his colleagues from Hunan University in Changsha,
China used an electrochemical reaction to develop a
method of urea synthesis. Its synthetic route, as
shown in Figure 5, directly combines nitrogen, CO
2
,
and water to form urea at ambient temperature and
pressure (American Chemical Society, 2021). While
this process is still in its preliminary stage, it offers a
possibility of producing urea fertilizers with lower
energy consumption rates and higher yields.
4 CONCLUSION
The simple organic compound urea has had an
unbelievable impact on the scientific community. The
urea cycle was also the first metabolic cycle
discovered by Krebs and Henseleit, which was even
earlier than their renowned Krebs (tricarboxylic)
cycle. As French chemist Louis Pasteur once said,
“Chance favors only the prepared mind” (Gibbons,
2013). In such a vast field of organic chemistry,
serendipitous discoveries happen when one is ready
to recognize. Wöhler was able to identify the
isomerization of ammonia to urea because he was
familiar with the compound from studying medicine
before (Shampo, 1985). Without Wöhler’s synthesis,
the idea of producing organic compounds from
inorganic ones would not even exist.
The transformative understanding of urea
synthesis now seeps into people’s everyday lives,
most prominently in fertilizers that pillar the
agricultural system worldwide. What may the next
step be? Perhaps a more resource and energy efficient
form of synthesis may be witnessed and fully
developed in the near future.
REFERENCES
American Chemical Society. (2021). Molecule of the week:
Urea. https://www.acs.org/content/acs/en/molecule-of-
the-week/archive/u/urea.html.
Ah Mew, N., Simpson, K.L., Gropman, A.L., et al. (2003)
Urea Cycle Disorders Overview. In: Adam, M.P.,
Ardinger, H.H., Pagon, R.A., et al., (Eds).
GeneReviews, University of Washington, Seattle,
Figure 1. [The urea cycle].
https://www.ncbi.nlm.nih.gov/sites/books/NBK1217/fi
gure/ucd-overview.F1/
Barmore, W., Azad, F., Stone, W.L. (2021). Physiology,
Urea Cycle. In: StatPearls [Internet]. Treasure Island
(FL): StatPearls Publishing;
https://www.ncbi.nlm.nih.gov/books/NBK513323/
Boerner, L.K. (2019). Industrial ammonia production emits
more CO
2
than any other chemical-making reactions.
Chemists want to change that. American Chemical
Society. https://cen.acs.org/environment/green-
chemistry/Industrial-ammonia-production-emits-
CO2/97/i24
Bradley, D. (2018). Solving urea's solubility problem.
ChemViews.
https://doi.org/10.1002/chemv.201800028
ICBB 2022 - International Conference on Biotechnology and Biomedicine
438

Carr, S. M. (2018). The Wöhler Synthesis of Urea (1828).
https://www.mun.ca/biology/scarr/4270_Wohler_Synt
hesis_of_Urea.html
Chen. C., et al. (2020). Coupling N2 and CO2 in H2O to
synthesize urea under ambient conditions. Nature
Chemistry. 12: 717–724.
Cheriyedath, S. (2019). What is the Urea Cycle? News
Medical. https://www.news-medical.net/health/What-
is-the-Urea-Cycle.aspx
Copplestone, J.C., Kirk, C.M., (2017). Ammonia and Urea
Production, New Zealand Institute of Chemistry,
https://nzic.org.nz/app/uploads/2017/10/1A.pdf
Eknoyan, G. (2017). A History of Uremia Research. J Ren
Nutr., 27(6): 449-452.
Friedmann, H. (1997). From Friedrich Wohler’s Urine to
Eduard Buchner’s Alcohol. In: Cornish-Bawden, A.,
(Eds.), New Beer in an Old Bottle: Eduard Buchner and
the Growth of Biochemical Knowledge, Universitat de
València, Valencia. pp. 67–122.
Gibbons, G. H. (2013). Serendipity and the Prepared Mind:
An NHLBI Intramural Researcher's Breakthrough
Observations. National Heart, Lung and Blood
Institute. https://www.nhlbi.nih.gov/directors-
messages/serendipity-and-the-prepared-mind
Heitmann, John Alfred. (1989). Friedrich Wöhler. In: Great
Lives From History: Renaissance to 1900. Vol. 5.
Salem Press, Pasadena. pp. 2252-2256.
Kyriakou, V., Garagounis, I., Vourros, A., Vasileiou, E., &
Stoukides, M. (2020). An electrochemical haber-bosch
process. Joule, 4(1): 142-158.
National Center for Biotechnology Information. (2021).
PubChem Compound Summary for CID 1176, Urea.
https://pubchem.ncbi.nlm.nih.gov/compound/Urea.
Raine, D. N. (1973). Book Review: Herman Boerhaave.
The Man and His Work. Annals of Clinical
Biochemistry, 10(1–6): 126-126.
Rabinovich, Daniel (2007). Wöhler’s Masterpiece.
Chemistry International -- Newsmagazine for IUPAC,
29(5): 3-3.
Sandhu, B., Sinha, A.S., Desper, J., Aakeröy, C.B. (2018).
Modulating the physical properties of solid forms of
urea using co-crystallization technology, Chem.
Commun., 37: 4657-4660
Shampo, M.A., Kyle, R.A. Early German Physician First to
Synthesize Urea. From: MZincke H, Engen DE,
Henning KM, McDonald MW. (1985). Treatment of
renal cell carcinoma by in situ partial nephrectomy and
extracorporeal operation with autotransplantation.
Mayo Clin Proc. 60(10):651-62.
The History of Urea and Its Use in the Modern Fertilizer Industry
439
